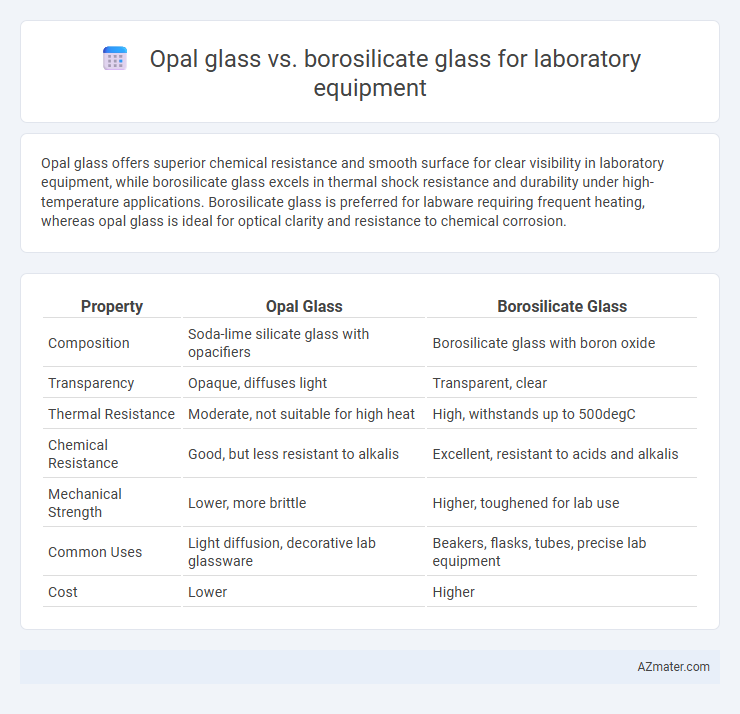
Opal glass offers superior chemical resistance and smooth surface for clear visibility in laboratory equipment, while borosilicate glass excels in thermal shock resistance and durability under high-temperature applications. Borosilicate glass is preferred for labware requiring frequent heating, whereas opal glass is ideal for optical clarity and resistance to chemical corrosion.
Table of Comparison
| Property | Opal Glass | Borosilicate Glass |
|---|---|---|
| Composition | Soda-lime silicate glass with opacifiers | Borosilicate glass with boron oxide |
| Transparency | Opaque, diffuses light | Transparent, clear |
| Thermal Resistance | Moderate, not suitable for high heat | High, withstands up to 500degC |
| Chemical Resistance | Good, but less resistant to alkalis | Excellent, resistant to acids and alkalis |
| Mechanical Strength | Lower, more brittle | Higher, toughened for lab use |
| Common Uses | Light diffusion, decorative lab glassware | Beakers, flasks, tubes, precise lab equipment |
| Cost | Lower | Higher |
Introduction to Laboratory Glassware Materials
Opal glass and borosilicate glass are common materials used in laboratory glassware, each with distinct properties suited for specific applications. Opal glass is valued for its diffused light transmission and chemical resistance, making it ideal for containers requiring opacity and aesthetic appeal. Borosilicate glass, known for its superior thermal resistance, low thermal expansion, and high durability, is preferred for heat-intensive laboratory processes such as heating, mixing, and storage of reactive chemicals.
What is Opal Glass?
Opal glass is a type of glass characterized by its milky, opaque white appearance, primarily used in laboratory equipment for its ability to diffuse light and provide uniform illumination. Composed mainly of silica, soda, and lime with added opacifiers like tin oxide or bone ash, opal glass offers excellent chemical resistance and moderate thermal stability but lower mechanical strength compared to borosilicate glass. Unlike borosilicate glass, which excels in thermal shock resistance and high-temperature applications, opal glass is preferred for applications requiring light diffusion and aesthetic appeal rather than extreme thermal durability.
What is Borosilicate Glass?
Borosilicate glass, known for its exceptional thermal resistance and chemical durability, is widely used in laboratory equipment due to its ability to withstand high temperatures and resist chemical corrosion. Unlike opal glass, which is typically opaque and mainly used for light diffusion, borosilicate glass offers superior transparency and mechanical strength, making it ideal for precise scientific experiments. Its low coefficient of thermal expansion minimizes thermal shock risks, ensuring reliability and safety in rigorous laboratory environments.
Chemical Resistance Comparison
Borosilicate glass exhibits superior chemical resistance compared to opal glass, particularly against strong acids, bases, and solvents commonly used in laboratory settings. Opal glass, primarily composed of soda-lime glass with added opacifiers, is more susceptible to chemical attack and etching, limiting its durability under harsh chemical exposure. Laboratories prefer borosilicate glass for its low reactivity, thermal stability, and ability to maintain integrity during repeated chemical processes.
Thermal Properties and Shock Resistance
Borosilicate glass offers superior thermal resistance with a low coefficient of thermal expansion (approximately 3.3 x 10^-6 /degC), enabling it to withstand rapid temperature changes and thermal shock without cracking. Opal glass, characterized by its milky, opaque appearance, has a higher thermal expansion coefficient and lower shock resistance, making it less suitable for high-temperature or sudden temperature fluctuation applications. Laboratories favor borosilicate glass for equipment like beakers and flasks exposed to direct heating due to its enhanced durability and reliability under thermal stress.
Transparency and Optical Clarity
BorosiIicate glass offers superior transparency and optical clarity, making it ideal for laboratory equipment requiring precise visual observations. Opal glass provides a diffused, opaque surface that reduces glare but limits visibility of contents. Laboratories prioritize borosilicate glass when clear, unobstructed viewing is essential for accurate measurements and reactions.
Mechanical Durability and Strength
Borosilicate glass exhibits superior mechanical durability and strength compared to opal glass, making it more resistant to thermal shock and mechanical stress in laboratory environments. Its low coefficient of thermal expansion reduces the risk of cracking under rapid temperature changes, a critical feature for high-precision lab equipment. Opal glass, while offering aesthetic diffuse light properties, lacks the robust strength and durability essential for demanding laboratory applications.
Cost Differences and Budget Considerations
Opal glass laboratory equipment generally costs less than borosilicate glass due to simpler manufacturing processes and lower raw material expenses. Borosilicate glass provides superior thermal resistance and chemical durability, resulting in higher initial costs but extended lifespan, influencing overall budget considerations for lab setups. Choosing between them requires balancing upfront expenditure with long-term performance and replacement frequency for cost-effective laboratory operations.
Typical Applications in Laboratory Settings
Opal glass is commonly used in laboratory equipment for items requiring UV protection and diffused light transmission, such as filter holders, crucibles, and certain types of glassware for photometric analysis. Borosilicate glass is preferred in applications demanding high thermal resistance and chemical durability, including beakers, test tubes, and condensers subjected to rapid temperature changes and aggressive chemicals. Both materials play critical roles in laboratory settings, with opal glass enhancing visibility and safety in light-sensitive procedures and borosilicate ensuring longevity and reliability in rigorous experimental environments.
Choosing the Right Glass for Your Lab Equipment
Opal glass offers excellent light diffusion and chemical resistance, making it suitable for applications requiring uniform light transmission and moderate thermal stability. Borosilicate glass features superior thermal shock resistance and durability, ideal for high-temperature experiments and harsh chemical environments in laboratories. Selecting the right glass depends on the specific lab requirements, with borosilicate preferred for heat-intensive tasks and opal glass chosen for light-sensitive processes.
Infographic: Opal glass vs Borosilicate glass for Laboratory equipment
 azmater.com
azmater.com