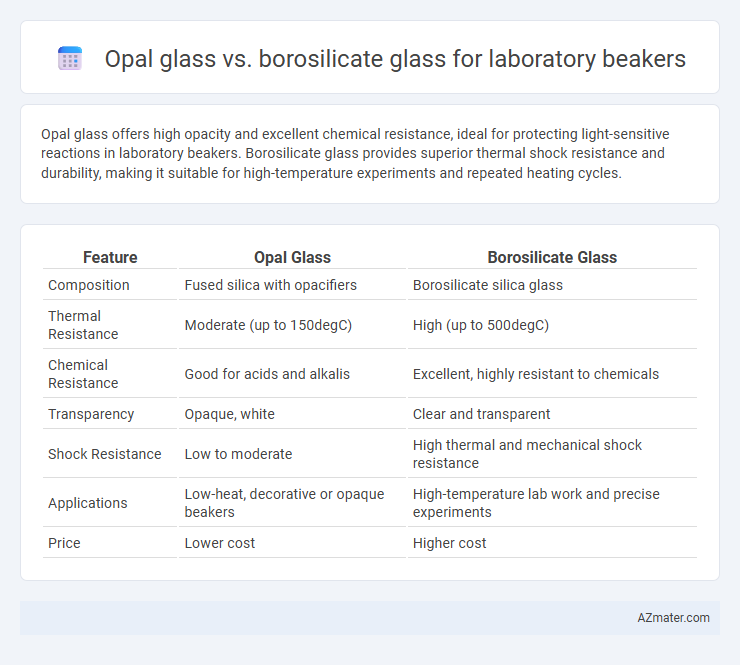
Opal glass offers high opacity and excellent chemical resistance, ideal for protecting light-sensitive reactions in laboratory beakers. Borosilicate glass provides superior thermal shock resistance and durability, making it suitable for high-temperature experiments and repeated heating cycles.
Table of Comparison
| Feature | Opal Glass | Borosilicate Glass |
|---|---|---|
| Composition | Fused silica with opacifiers | Borosilicate silica glass |
| Thermal Resistance | Moderate (up to 150degC) | High (up to 500degC) |
| Chemical Resistance | Good for acids and alkalis | Excellent, highly resistant to chemicals |
| Transparency | Opaque, white | Clear and transparent |
| Shock Resistance | Low to moderate | High thermal and mechanical shock resistance |
| Applications | Low-heat, decorative or opaque beakers | High-temperature lab work and precise experiments |
| Price | Lower cost | Higher cost |
Introduction to Laboratory Glassware Materials
Opal glass and borosilicate glass differ significantly in laboratory applications due to their material properties. Opal glass, characterized by its milky white appearance, offers good chemical resistance but lower thermal shock resistance compared to borosilicate glass. Borosilicate glass, renowned for its exceptional thermal stability and durability, is the preferred choice for laboratory beakers requiring heat tolerance and resistance to chemical corrosion.
What is Opal Glass?
Opal glass, also known as milk glass, is a type of borosilicate glass that is treated to have a white, opaque appearance, making it ideal for laboratory beakers used in light-sensitive experiments. It offers excellent chemical resistance and thermal stability similar to clear borosilicate glass but provides the added benefit of light diffusion to protect photosensitive substances. This makes opal glass beakers a preferred choice in labs where reducing light exposure is critical.
What is Borosilicate Glass?
Borosilicate glass is a type of laboratory glass known for its exceptional thermal resistance and chemical durability, making it ideal for beakers used in high-temperature experiments. It contains boron trioxide, which reduces thermal expansion and increases resistance to thermal shock compared to standard glass. This composition allows borosilicate beakers to withstand rapid temperature changes and acidic or alkaline substances without cracking or degrading.
Key Material Properties Compared
Opal glass offers excellent chemical resistance and diffuses light, making it ideal for applications requiring translucency and moderate thermal resistance. Borosilicate glass exhibits superior thermal shock resistance and high mechanical strength, allowing it to withstand rapid temperature changes common in laboratory settings. Both materials resist corrosion, but borosilicate's low thermal expansion coefficient enhances durability under extreme heating and cooling cycles.
Thermal Resistance: Opal vs Borosilicate
Borosilicate glass offers superior thermal resistance with a high coefficient of thermal shock resistance, allowing it to withstand rapid temperature changes up to approximately 450degC without cracking. Opal glass, while heat-resistant and opaque, has significantly lower thermal shock tolerance and is more prone to fracture under sudden temperature fluctuations. Laboratories prioritize borosilicate glass beakers for applications involving extreme temperature variations due to its durability and reliability in thermal resistance compared to opal glass.
Chemical Durability in Laboratory Use
Borosilicate glass demonstrates superior chemical durability compared to opal glass, resisting corrosion and chemical attack from acids, bases, and solvents commonly used in laboratory settings. Opal glass, while visually opaque and aesthetically pleasing, often lacks the chemical resistance necessary for prolonged exposure to aggressive reagents. Laboratories prioritize borosilicate glass beakers for applications requiring high thermal and chemical stability to ensure accuracy and safety during experiments.
Transparency and Visibility Differences
Opal glass laboratory beakers exhibit lower transparency due to their milky, opaque appearance, which diffuses light and reduces clear visibility of contents. Borosilicate glass offers high transparency, enabling precise observation of reactions and fluid levels without distortion. The enhanced clarity of borosilicate glass makes it ideal for applications requiring detailed visual monitoring in lab environments.
Cost and Availability Considerations
Opal glass beakers generally cost more than borosilicate glass due to their specialized manufacturing process that imparts a translucent appearance, making them less commonly stocked by suppliers. Borosilicate glass beakers are widely available and economically priced, benefiting from extensive demand and mass production in laboratory settings. Laboratories prioritizing cost-efficiency and ease of procurement often prefer borosilicate glass for standard beaker applications.
Suitability for Different Laboratory Applications
Opal glass laboratory beakers offer excellent visual opacity, making them suitable for applications requiring light-sensitive reactions or easy visibility of liquid levels. Borosilicate glass beakers provide superior thermal resistance and chemical durability, ideal for high-temperature experiments and handling aggressive chemicals. Selecting between opal and borosilicate glass depends on the specific laboratory application needs, such as heat tolerance, chemical compatibility, and observational requirements.
Conclusion: Choosing the Right Glass for Your Beakers
Opal glass is ideal for laboratory beakers requiring high thermal resistance and chemical durability, offering opacity that protects light-sensitive reactions. Borosilicate glass provides exceptional thermal shock resistance and transparency, making it suitable for experiments needing visibility and frequent temperature changes. Selecting the right glass depends on experimental needs: opt for opal glass for light-sensitive applications and borosilicate for general-purpose, high-temperature, and visibility-demanding tasks.
Infographic: Opal glass vs Borosilicate glass for Laboratory beaker
 azmater.com
azmater.com