Borosilicate glass offers superior thermal resistance and chemical durability compared to soda lime glass, making it ideal for laboratory apparatus exposed to high temperatures and corrosive chemicals. Soda lime glass is less expensive but more prone to thermal shock and chemical degradation, limiting its use in demanding laboratory applications.
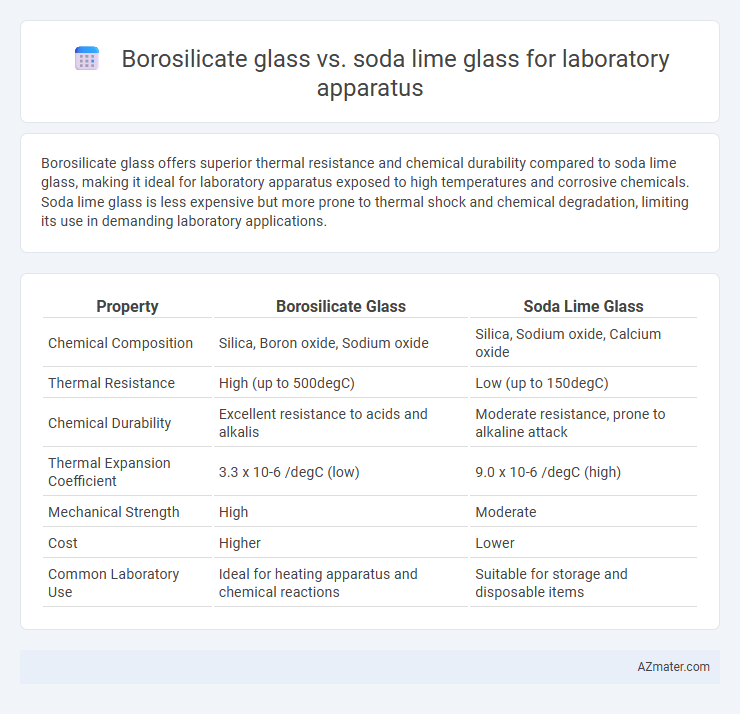
Table of Comparison
| Property | Borosilicate Glass | Soda Lime Glass |
|---|---|---|
| Chemical Composition | Silica, Boron oxide, Sodium oxide | Silica, Sodium oxide, Calcium oxide |
| Thermal Resistance | High (up to 500degC) | Low (up to 150degC) |
| Chemical Durability | Excellent resistance to acids and alkalis | Moderate resistance, prone to alkaline attack |
| Thermal Expansion Coefficient | 3.3 x 10-6 /degC (low) | 9.0 x 10-6 /degC (high) |
| Mechanical Strength | High | Moderate |
| Cost | Higher | Lower |
| Common Laboratory Use | Ideal for heating apparatus and chemical reactions | Suitable for storage and disposable items |
Introduction to Laboratory Glassware
Borosilicate glass offers superior thermal resistance and chemical durability compared to soda lime glass, making it ideal for laboratory apparatus subjected to high temperatures and corrosive substances. Soda lime glass, while more cost-effective, is less resistant to thermal shock and chemical attack, limiting its use to less demanding laboratory applications. The choice between borosilicate and soda lime glass impacts the reliability and safety of laboratory experiments, particularly in heating and chemical exposure scenarios.
Composition of Borosilicate Glass
Borosilicate glass used in laboratory apparatus contains approximately 80% silica (SiO2) and 13% boric oxide (B2O3), which imparts superior thermal resistance and chemical durability compared to soda lime glass, primarily composed of silica, sodium oxide (Na2O), and calcium oxide (CaO). The inclusion of boric oxide in borosilicate glass minimizes thermal expansion coefficients to approximately 3.3 x 10-6 /degC, enabling it to withstand rapid temperature changes without cracking. These compositional differences make borosilicate glass ideal for precise scientific applications requiring high resistance to thermal shock and chemical corrosion.
Composition of Soda Lime Glass
Soda lime glass, primarily composed of about 70-75% silica (SiO2), 12-15% sodium oxide (Na2O), and 10-15% calcium oxide (CaO), is commonly used in laboratory apparatus due to its low cost and ease of manufacture. Borosilicate glass differs significantly by incorporating approximately 12-13% boron trioxide (B2O3), which enhances thermal resistance and chemical durability compared to the soda lime glass's simpler composition. While soda lime glass offers adequate clarity and is suitable for basic labware, borosilicate glass is preferred for applications requiring superior thermal shock resistance and chemical inertness.
Thermal Resistance Comparison
Borosilicate glass exhibits superior thermal resistance with a coefficient of thermal expansion around 3.3 x 10^-6 /degC, enabling it to withstand rapid temperature changes without cracking, making it ideal for laboratory apparatus exposed to heat. In contrast, soda lime glass has a higher coefficient of thermal expansion, approximately 9 x 10^-6 /degC, which limits its ability to endure thermal shock and reduces its suitability for high-temperature lab applications. Laboratory glassware like beakers, flasks, and test tubes often utilize borosilicate glass due to its robustness in thermal processes such as heating and cooling cycles.
Chemical Resistance: Borosilicate vs Soda Lime
Borosilicate glass exhibits superior chemical resistance compared to soda lime glass, making it ideal for laboratory apparatus exposed to strong acids, bases, and solvents. Soda lime glass is more susceptible to chemical attack, leading to surface degradation and potential contamination. Borosilicate's low alkali content enhances its durability and purity in chemical applications.
Mechanical Strength and Durability
Borosilicate glass exhibits superior mechanical strength and enhanced durability compared to soda lime glass, making it the preferred choice for laboratory apparatus subjected to thermal and mechanical stress. Its low thermal expansion coefficient allows it to withstand rapid temperature changes without cracking, whereas soda lime glass is more prone to breakage under similar conditions. Borosilicate also resists chemical corrosion better, prolonging the lifespan of laboratory equipment in rigorous experimental environments.
Cost Differences in Laboratory Applications
Borosilicate glass laboratory apparatus typically costs higher than soda lime glass due to its superior chemical resistance, thermal stability, and durability under thermal shock. Soda lime glass is more economical, making it suitable for disposable or routine applications where extreme chemical or thermal conditions are not encountered. Laboratories often balance cost versus performance needs, choosing borosilicate glass for precision experiments and soda lime glass for budget-conscious general use.
Common Laboratory Uses for Each Glass Type
Borosilicate glass is widely used in laboratory apparatus such as beakers, test tubes, and condensers due to its exceptional thermal resistance and chemical durability, making it ideal for heating and handling corrosive substances. Soda lime glass, while less resistant to thermal shock, is commonly preferred for disposable or less demanding applications like petri dishes and storage containers because of its cost-effectiveness and adequate chemical resistance. Laboratories often select borosilicate glass for precision experiments requiring high-temperature stability and soda lime glass for routine, low-stress tasks to balance performance and budget.
Safety Considerations and Breakage
Borosilicate glass offers superior thermal resistance and chemical durability compared to soda lime glass, minimizing the risk of breakage under rapid temperature changes or exposure to corrosive substances in laboratory settings. Soda lime glass is more prone to cracking and shattering when subjected to thermal stress or mechanical impact, which can compromise laboratory safety. Selecting borosilicate glass for laboratory apparatus enhances safety by reducing breakage incidents and ensuring consistent performance during experiments.
Choosing the Right Glass for Your Laboratory
Borosilicate glass offers superior thermal resistance and chemical durability compared to soda lime glass, making it ideal for laboratory apparatus subjected to rapid temperature changes and corrosive chemicals. Soda lime glass is more cost-effective but less resistant to thermal shock and chemical exposure, limiting its suitability for high-precision or harsh laboratory environments. Selecting borosilicate glass ensures enhanced safety, longevity, and reliability for critical lab applications, especially when working with high temperatures or aggressive reagents.
Infographic: Borosilicate glass vs Soda lime glass for Laboratory apparatus
 azmater.com
azmater.com