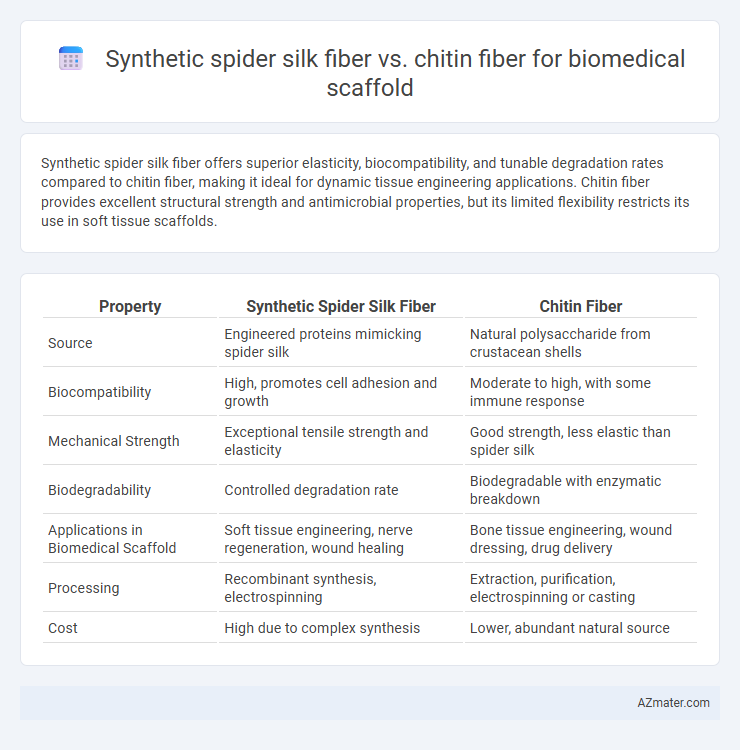
Synthetic spider silk fiber offers superior elasticity, biocompatibility, and tunable degradation rates compared to chitin fiber, making it ideal for dynamic tissue engineering applications. Chitin fiber provides excellent structural strength and antimicrobial properties, but its limited flexibility restricts its use in soft tissue scaffolds.
Table of Comparison
| Property | Synthetic Spider Silk Fiber | Chitin Fiber |
|---|---|---|
| Source | Engineered proteins mimicking spider silk | Natural polysaccharide from crustacean shells |
| Biocompatibility | High, promotes cell adhesion and growth | Moderate to high, with some immune response |
| Mechanical Strength | Exceptional tensile strength and elasticity | Good strength, less elastic than spider silk |
| Biodegradability | Controlled degradation rate | Biodegradable with enzymatic breakdown |
| Applications in Biomedical Scaffold | Soft tissue engineering, nerve regeneration, wound healing | Bone tissue engineering, wound dressing, drug delivery |
| Processing | Recombinant synthesis, electrospinning | Extraction, purification, electrospinning or casting |
| Cost | High due to complex synthesis | Lower, abundant natural source |
Introduction to Synthetic Spider Silk and Chitin Fibers
Synthetic spider silk fibers exhibit exceptional tensile strength, biocompatibility, and biodegradability, making them ideal candidates for advanced biomedical scaffolds that promote tissue regeneration. Chitin fibers, derived from crustacean shells, offer inherent antimicrobial properties, biodegradability, and structural similarity to glycosaminoglycans found in human tissue, supporting cell adhesion and proliferation. Both materials provide unique biomimetic qualities, with synthetic spider silk delivering superior mechanical performance and chitin fibers enhancing biological interaction in scaffold design.
Biomaterial Properties: Strength, Flexibility, and Biocompatibility
Synthetic spider silk fiber exhibits exceptional tensile strength and remarkable flexibility, closely mimicking natural silk, making it ideal for durable and elastic biomedical scaffolds. Chitin fiber offers robust mechanical support with inherent biocompatibility and biodegradability, supporting cell adhesion and promoting tissue regeneration. Both biomaterials demonstrate high biocompatibility, but synthetic spider silk's combination of strength, flexibility, and minimal immune response often provides superior performance in dynamic tissue engineering applications.
Molecular Structure Comparison
Synthetic spider silk fiber exhibits a beta-sheet-rich molecular structure, providing exceptional tensile strength and elasticity ideal for biomedical scaffolds. In contrast, chitin fiber consists of a polysaccharide chain with acetylated amino groups forming a crystalline nanofiber network, offering biocompatibility and biodegradability but lower mechanical strength. The hierarchical organization of synthetic spider silk at the molecular level enables superior durability and flexibility compared to the primarily rigid and brittle molecular arrangement of chitin fibers.
Sourcing and Production Methods
Synthetic spider silk fiber is produced through recombinant DNA technology, enabling scalable and controlled synthesis in microbial hosts like E. coli or yeast, ensuring consistent quality and mechanical properties. Chitin fiber, derived from the exoskeletons of crustaceans and insects, requires extensive extraction and purification processes involving demineralization and deproteination to obtain biomedical-grade material. The synthetic approach offers greater customization and batch-to-batch reproducibility compared to the natural variability and sourcing limitations inherent in chitin fiber extraction.
Mechanical Performance in Scaffolding Applications
Synthetic spider silk fiber demonstrates exceptional tensile strength and elasticity, closely mimicking the native extracellular matrix, which enhances cellular adhesion and proliferation in biomedical scaffolds. Chitin fiber offers inherent biocompatibility and biodegradability but generally exhibits lower mechanical robustness compared to synthetic spider silk, potentially limiting scaffold durability under physiological loads. The superior mechanical performance of synthetic spider silk makes it more suitable for load-bearing scaffold applications requiring long-term structural integrity.
Degradability and Bioactivity in Biomedical Environments
Synthetic spider silk fiber exhibits controlled biodegradability and excellent bioactivity, making it an ideal candidate for biomedical scaffolds that require gradual resorption and tissue integration. Chitin fiber offers natural antimicrobial properties and biocompatibility but often demonstrates slower degradation rates, which can impact scaffold remodeling and cellular responses in vivo. The tunable degradation kinetics and superior cell adhesion properties of synthetic spider silk provide enhanced performance over chitin for dynamic tissue engineering applications.
Cell Adhesion and Proliferation on Scaffolds
Synthetic spider silk fiber exhibits superior cell adhesion and proliferation properties compared to chitin fiber when used in biomedical scaffolds due to its excellent biocompatibility and tunable mechanical strength. The molecular structure of spider silk promotes enhanced integrin binding sites, facilitating improved cell attachment and growth, while chitin fibers often require chemical modification to achieve comparable bioactivity. Studies demonstrate that synthetic spider silk scaffolds support faster proliferation rates and uniform cell distribution, critical for tissue regeneration applications.
Immunogenicity and Safety Profiles
Synthetic spider silk fiber demonstrates superior biocompatibility and lower immunogenicity compared to chitin fiber, reducing the risk of inflammatory responses in biomedical scaffolds. Chitin fibers, while biodegradable and promoting cell adhesion, can trigger stronger immune reactions due to residual endotoxins and their polysaccharide nature. Clinical studies emphasize synthetic spider silk's safer profile for long-term implantation, highlighting minimal immunogenicity and a stable, non-toxic degradation process ideal for tissue engineering.
Current Biomedical Applications and Research
Synthetic spider silk fiber exhibits exceptional tensile strength, elasticity, and biocompatibility, making it a prominent material for wound healing, nerve regeneration, and tissue engineering scaffolds. Chitin fiber, derived from crustacean shells, offers inherent biodegradability, antimicrobial properties, and supports cell adhesion, frequently utilized in wound dressings and bone tissue scaffolds. Current research emphasizes synthetic spider silk's molecular customization for enhanced mechanical performance, while chitin's polysaccharide structure is being engineered to improve bioactivity and controlled degradation rates in biomedical applications.
Future Outlook and Innovations in Fiber-Based Scaffolds
Synthetic spider silk fiber offers superior mechanical strength, biocompatibility, and tunable degradation rates compared to chitin fiber, making it a promising candidate for next-generation biomedical scaffolds. Innovations in fiber-based scaffolds focus on integrating synthetic spider silk with nanotechnology and bioactive molecules to enhance tissue regeneration and cellular integration. Future outlook emphasizes scalable production methods and hybrid scaffold designs combining synthetic spider silk with chitin to leverage their complementary biological properties for advanced wound healing and tissue engineering applications.
Infographic: Synthetic spider silk fiber vs Chitin fiber for Biomedical scaffold
 azmater.com
azmater.com