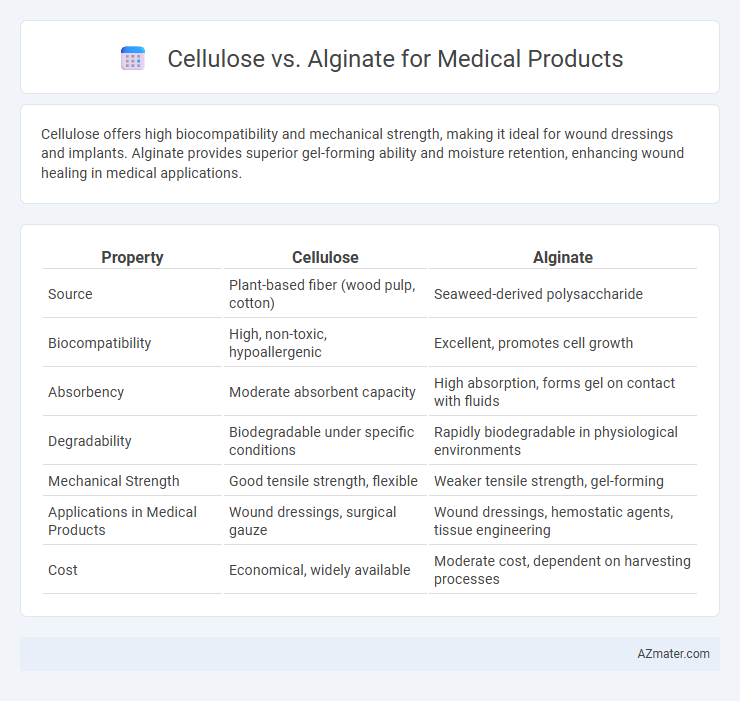
Cellulose offers high biocompatibility and mechanical strength, making it ideal for wound dressings and implants. Alginate provides superior gel-forming ability and moisture retention, enhancing wound healing in medical applications.
Table of Comparison
| Property | Cellulose | Alginate |
|---|---|---|
| Source | Plant-based fiber (wood pulp, cotton) | Seaweed-derived polysaccharide |
| Biocompatibility | High, non-toxic, hypoallergenic | Excellent, promotes cell growth |
| Absorbency | Moderate absorbent capacity | High absorption, forms gel on contact with fluids |
| Degradability | Biodegradable under specific conditions | Rapidly biodegradable in physiological environments |
| Mechanical Strength | Good tensile strength, flexible | Weaker tensile strength, gel-forming |
| Applications in Medical Products | Wound dressings, surgical gauze | Wound dressings, hemostatic agents, tissue engineering |
| Cost | Economical, widely available | Moderate cost, dependent on harvesting processes |
Introduction to Cellulose and Alginate in Medical Applications
Cellulose, a natural polymer derived from plant cell walls, is extensively utilized in medical products due to its biocompatibility, biodegradability, and non-toxicity, making it ideal for wound dressings, drug delivery systems, and tissue engineering. Alginate, extracted from brown seaweed, is highly valued in medical applications for its excellent gel-forming properties, ability to maintain a moist wound environment, and hemostatic capabilities, commonly used in wound care and controlled drug release. Both cellulose and alginate offer unique advantages tailored to specific biomedical applications, with cellulose providing structural support and alginate enabling effective moisture management and bioactivity.
Chemical Structure and Properties Comparison
Cellulose is a linear polysaccharide composed of b-1,4-linked D-glucose units forming strong hydrogen bonds that provide high tensile strength and insolubility in water, making it ideal for structural support in medical products. Alginate consists of linear copolymers of b-D-mannuronic acid and a-L-guluronic acid residues with carboxyl and hydroxyl groups, allowing ion exchange and gel-forming properties essential for wound dressings and drug delivery. The difference in chemical structure results in cellulose's rigidity and stability contrasting with alginate's hydrophilicity and biocompatible gelation capacity, influencing their respective functionalities in biomedical applications.
Sources and Extraction Methods
Cellulose, derived primarily from plant cell walls such as wood pulp and cotton fibers, undergoes chemical or mechanical extraction processes including pulping and bleaching to achieve high purity for medical applications. Alginate is extracted from brown seaweed species like Laminaria and Ascophyllum through alkaline treatment followed by precipitation, preserving its biocompatibility and gel-forming properties essential for wound dressings and drug delivery. Both materials require stringent purification steps to remove impurities and maintain biocompatibility, but alginate's marine origin offers unique hemostatic and bioadhesive advantages compared to cellulose's robust structural cellulose molecules.
Biocompatibility and Safety Profiles
Cellulose derivatives and alginate are both widely used in medical products due to their excellent biocompatibility and safety profiles. Cellulose is known for its inert nature, minimal immunogenicity, and strong structural stability, making it ideal for wound dressings and drug delivery systems. Alginate, derived from brown seaweed, offers superior hemostatic properties and promotes cell adhesion and proliferation while maintaining biodegradability and minimal cytotoxicity, favoring applications like tissue engineering and regenerative medicine.
Mechanical Strength and Stability
Cellulose-based materials exhibit high tensile strength and excellent mechanical durability, making them suitable for medical products requiring robust structural integrity. Alginate, derived from brown seaweed, shows superior gel-forming ability and biocompatibility but generally has lower mechanical strength compared to cellulose, limiting its use in load-bearing applications. Stability of cellulose materials under physiological conditions surpasses alginate, which can degrade or dissolve in aqueous environments, affecting long-term performance in medical devices.
Applications in Wound Care Products
Cellulose and alginate are widely utilized in wound care products due to their biocompatibility and moisture retention properties. Cellulose-based dressings excel in providing a moist healing environment and are often used for light to moderately exuding wounds, promoting autolytic debridement. Alginate dressings, derived from brown seaweed, are highly absorbent and suitable for moderate to heavily exuding wounds, offering hemostatic properties and enhanced wound healing through gel formation upon contact with wound exudate.
Drug Delivery Systems: Cellulose vs Alginate
Cellulose and alginate are widely used polymers in drug delivery systems, offering distinct advantages based on their chemical structure and biocompatibility. Cellulose derivatives, such as hydroxypropyl methylcellulose, provide controlled drug release and mechanical stability, making them ideal for oral and topical formulations. Alginate's natural gel-forming ability and ion-exchange properties enable efficient encapsulation and targeted release, especially in wound dressings and injectable hydrogels for sustained therapeutic effects.
Cost-Effectiveness and Scalability
Cellulose offers high cost-effectiveness due to its abundant availability and low production costs, making it ideal for large-scale medical product manufacturing. Alginate, derived from brown seaweed, provides excellent biocompatibility but tends to have higher raw material costs and more complex extraction processes, which can limit scalability. Scaling up cellulose-based products benefits from a well-established supply chain, while alginate's variable seaweed harvests may affect consistent large-scale production.
Regulatory Considerations for Medical Use
Cellulose and alginate are both widely used in medical products, but they differ significantly in regulatory pathways due to their origin and processing. Cellulose, often derived from plant sources, is typically considered safe and inert, facilitating FDA clearance under medical device regulations such as 510(k) for wound dressings and drug delivery systems. Alginate, a biopolymer extracted from seaweed, faces stricter scrutiny because of variability in source material and potential for immune reactions, requiring thorough biocompatibility testing and compliance with ISO 10993 standards for medical applications.
Future Prospects and Innovations
Cellulose and alginate continue to drive innovations in medical products due to their biocompatibility and biodegradability. Future prospects highlight nanocellulose composites offering enhanced mechanical strength and controlled drug release, while alginate's tunable gelation properties enable advanced wound dressings and tissue engineering scaffolds. Emerging research focuses on hybrid materials combining cellulose and alginate to optimize functionality in regenerative medicine and targeted therapy applications.
Infographic: Cellulose vs Alginate for Medical product
 azmater.com
azmater.com