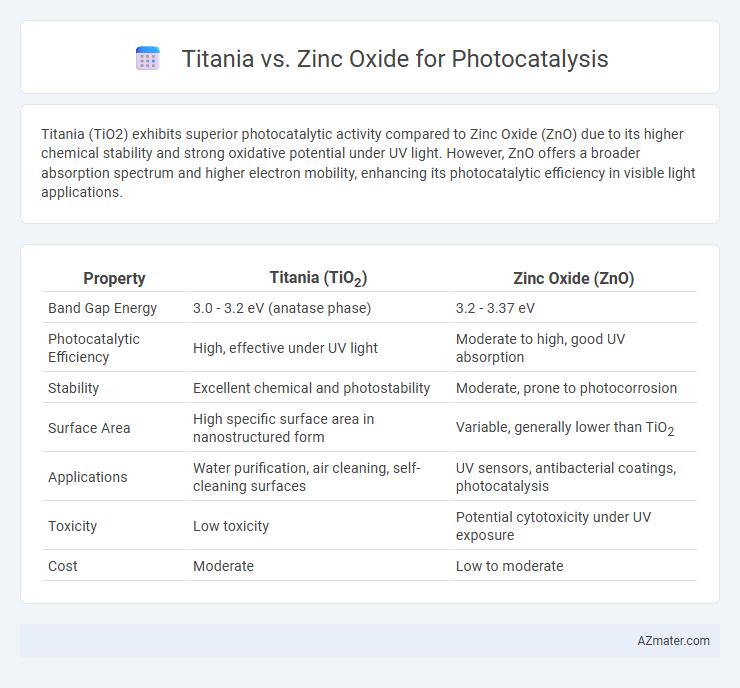
Titania (TiO2) exhibits superior photocatalytic activity compared to Zinc Oxide (ZnO) due to its higher chemical stability and strong oxidative potential under UV light. However, ZnO offers a broader absorption spectrum and higher electron mobility, enhancing its photocatalytic efficiency in visible light applications.
Table of Comparison
| Property | Titania (TiO2) | Zinc Oxide (ZnO) |
|---|---|---|
| Band Gap Energy | 3.0 - 3.2 eV (anatase phase) | 3.2 - 3.37 eV |
| Photocatalytic Efficiency | High, effective under UV light | Moderate to high, good UV absorption |
| Stability | Excellent chemical and photostability | Moderate, prone to photocorrosion |
| Surface Area | High specific surface area in nanostructured form | Variable, generally lower than TiO2 |
| Applications | Water purification, air cleaning, self-cleaning surfaces | UV sensors, antibacterial coatings, photocatalysis |
| Toxicity | Low toxicity | Potential cytotoxicity under UV exposure |
| Cost | Moderate | Low to moderate |
Introduction to Photocatalysis
Photocatalysis utilizes semiconductor materials like Titania (TiO2) and Zinc Oxide (ZnO) to accelerate chemical reactions under light exposure. Titania is widely favored for its strong oxidative power, chemical stability, and non-toxicity, making it effective in environmental remediation and self-cleaning applications. Zinc Oxide offers a comparable bandgap and high exciton binding energy, but its photocatalytic efficiency can be hindered by faster recombination of photo-generated charge carriers compared to Titania.
Overview of Titania (TiO₂) as a Photocatalyst
Titania (TiO2) is widely recognized for its exceptional photocatalytic properties due to its strong oxidizing power, chemical stability, and non-toxicity. Its bandgap of approximately 3.2 eV enables efficient absorption of UV light, facilitating the generation of electron-hole pairs that drive photodegradation of pollutants. The anatase phase of TiO2 exhibits superior photocatalytic activity compared to other polymorphs, making it a preferred material for environmental remediation and solar energy applications.
Overview of Zinc Oxide (ZnO) as a Photocatalyst
Zinc Oxide (ZnO) stands out as a highly efficient photocatalyst due to its wide bandgap of approximately 3.37 eV and high exciton binding energy of 60 meV, enabling strong UV light absorption and effective generation of electron-hole pairs. ZnO exhibits remarkable photocatalytic activity in degrading organic pollutants, splitting water, and antibacterial applications, attributed to its high surface area, chemical stability, and ease of synthesis into various nanostructures such as nanorods, nanoparticles, and nanosheets. Compared to Titania (TiO2), ZnO often demonstrates higher photocatalytic efficiency under UV illumination but faces challenges related to photocorrosion and narrower stability under prolonged light exposure.
Photocatalytic Mechanisms: Titania vs Zinc Oxide
Titania (TiO2) and Zinc Oxide (ZnO) both exhibit strong photocatalytic activities due to their wide bandgaps, with TiO2 typically around 3.2 eV and ZnO near 3.3 eV, enabling them to generate electron-hole pairs under UV light. In TiO2, the photocatalytic mechanism involves anatase phase favoring higher oxidative potential and longer electron-hole pair lifetimes, while ZnO often shows faster recombination rates but greater surface reactivity due to oxygen vacancies. Charge carrier separation efficiency, surface adsorption sites, and defect states critically influence the photocatalytic performance, with TiO2 generally excelling in environmental applications due to its superior stability and durability compared to ZnO.
Light Absorption and Band Gap Comparison
Titania (TiO2) and zinc oxide (ZnO) are widely studied photocatalysts due to their effective light absorption and suitable band gap energies. Titania typically exhibits a band gap of about 3.0-3.2 eV, allowing it to absorb ultraviolet light up to approximately 388 nm, whereas zinc oxide has a slightly wider band gap around 3.2-3.3 eV with UV absorption near 375 nm. The narrower band gap of titania can enhance visible light photocatalytic efficiency under solar irradiation, while zinc oxide's electronic properties provide higher exciton binding energy, influencing charge separation and photocatalytic activity.
Photocatalytic Efficiency: Experimental Studies
Experimental studies consistently demonstrate that titanium dioxide (TiO2) exhibits higher photocatalytic efficiency compared to zinc oxide (ZnO) due to its superior charge separation and stability under UV irradiation. TiO2's anatase phase, especially when doped or nanostructured, enhances reactive oxygen species generation, leading to more effective degradation of organic pollutants. Although ZnO shows comparable initial photocatalytic activity, its rapid photocorrosion significantly limits long-term efficiency in aqueous environments.
Stability and Durability Under Photocatalytic Conditions
Titania (TiO2) exhibits superior stability and durability under photocatalytic conditions due to its robust crystal structure and resistance to photocorrosion, maintaining performance over extended usage. Zinc oxide (ZnO), while effective in photocatalysis, often suffers from photocorrosion and degradation when exposed to UV light and reactive oxygen species, limiting its long-term stability. Advanced modifications to ZnO can enhance its durability, but TiO2 remains the preferred material for applications demanding sustained photocatalytic activity.
Environmental Applications: Water and Air Purification
Titania (TiO2) exhibits superior photocatalytic efficiency compared to zinc oxide (ZnO) in environmental applications due to its higher chemical stability and non-toxicity, making it ideal for water and air purification. TiO2 effectively degrades organic pollutants, volatile organic compounds (VOCs), and harmful bacteria under UV light, leading to improved water quality and cleaner air. Although ZnO has a similar bandgap and strong photocatalytic activity, its photocorrosion limits long-term use in harsh environmental conditions.
Advantages and Limitations of Titania and Zinc Oxide
Titania offers high chemical stability and strong oxidative power, making it highly effective for photocatalysis under UV light, but its wide band gap limits visible light absorption. Zinc oxide provides a similar band gap but exhibits higher electron mobility and diverse morphologies, enhancing photocatalytic efficiency, though it suffers from photocorrosion and lower stability in aqueous media. Both materials face challenges in visible light utilization, requiring modifications to improve solar-driven photocatalytic applications.
Future Prospects and Research Directions
Titania (TiO2) and Zinc Oxide (ZnO) remain at the forefront of photocatalysis research due to their distinct band gaps and charge carrier dynamics. Future prospects emphasize enhancing visible-light absorption through doping and heterojunction engineering to improve quantum efficiency and stability under operational conditions. Emerging research directions prioritize scalable synthesis methods, defect engineering, and hybrid nanostructures to expand their applicability in environmental remediation and solar energy conversion.
Infographic: Titania vs Zinc oxide for Photocatalysis
 azmater.com
azmater.com