Refractory materials exhibit high thermal stability and mechanical strength, making them ideal for catalyst supports in harsh environments. Ceramic oxides offer superior chemical resistance and surface area, enhancing catalytic activity and durability.
Table of Comparison
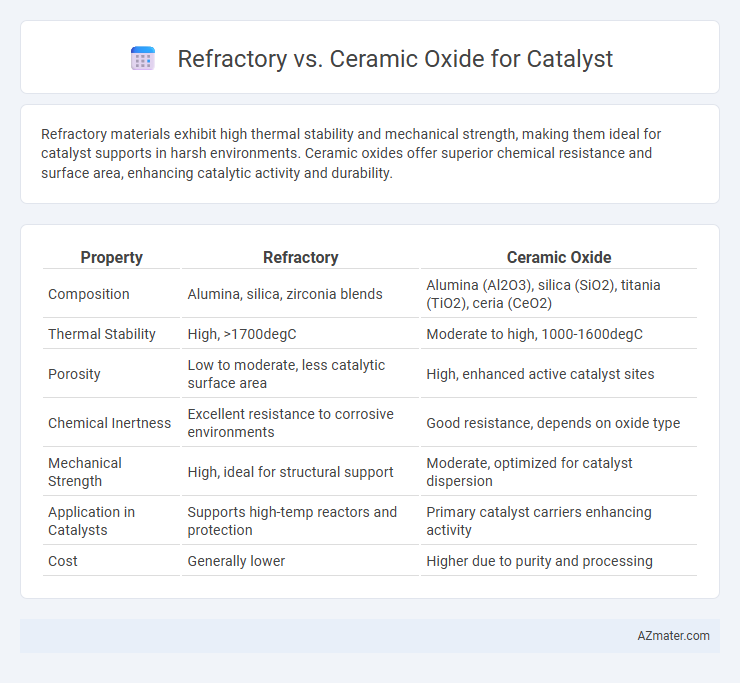
| Property | Refractory | Ceramic Oxide |
|---|---|---|
| Composition | Alumina, silica, zirconia blends | Alumina (Al2O3), silica (SiO2), titania (TiO2), ceria (CeO2) |
| Thermal Stability | High, >1700degC | Moderate to high, 1000-1600degC |
| Porosity | Low to moderate, less catalytic surface area | High, enhanced active catalyst sites |
| Chemical Inertness | Excellent resistance to corrosive environments | Good resistance, depends on oxide type |
| Mechanical Strength | High, ideal for structural support | Moderate, optimized for catalyst dispersion |
| Application in Catalysts | Supports high-temp reactors and protection | Primary catalyst carriers enhancing activity |
| Cost | Generally lower | Higher due to purity and processing |
Introduction to Catalyst Supports
Refractory materials and ceramic oxides serve as critical catalyst supports by providing thermal stability and mechanical strength in high-temperature reactions. Refractory supports, such as alumina and silica, offer excellent resistance to sintering and chemical degradation, maintaining catalyst dispersion and activity. Ceramic oxides like zirconia and titania enhance catalytic performance by providing unique surface properties and oxygen storage capacity, facilitating redox reactions and improving catalyst durability.
Overview of Refractory Oxides
Refractory oxides such as alumina, silica, and zirconia exhibit high thermal stability and mechanical strength, making them ideal supports for catalysts in high-temperature industrial processes. These materials provide excellent resistance to sintering and chemical poisoning, ensuring prolonged catalyst activity and durability. Their porous structure enables effective dispersion of active metal sites, enhancing catalytic efficiency in reactions like hydrocracking and oxidation.
Characteristics of Ceramic Oxides
Ceramic oxides exhibit high thermal stability, excellent chemical inertness, and strong oxygen ion conductivity, making them ideal for catalyst supports and active components in catalytic reactions. Their porous structure enhances surface area, promoting better catalyst dispersion and improved reaction efficiency. Common ceramic oxides such as alumina, zirconia, and ceria also provide resistance to sintering and mechanical wear, ensuring durability under harsh process conditions.
Thermal Stability Comparison
Refractory materials exhibit superior thermal stability compared to ceramic oxides, maintaining structural integrity at temperatures exceeding 1500degC, crucial for high-temperature catalytic processes. Ceramic oxides, such as alumina and zirconia, offer good thermal resistance up to approximately 1200degC but tend to sinter and lose active surface area under extreme conditions. Refractory catalysts ensure prolonged durability and performance in harsh thermal environments, making them preferable for industrial applications requiring sustained high-temperature stability.
Surface Area and Porosity Differences
Refractory materials typically exhibit lower surface area and limited porosity compared to ceramic oxides, which possess higher surface area and well-developed pore structures essential for catalytic activity. The enhanced porosity of ceramic oxides facilitates greater reactant diffusion and active site accessibility, improving catalyst efficiency. Surface area differences significantly influence catalytic performance, with ceramic oxides offering superior adsorption capacity and reaction interface due to their micro- and mesoporous architecture.
Chemical Resistance and Durability
Refractory materials exhibit superior chemical resistance and thermal stability, making them ideal for catalyst supports in high-temperature environments. Ceramic oxides, such as alumina and zirconia, offer enhanced durability through their exceptional resistance to acidic and basic conditions, maintaining structural integrity during catalytic reactions. The choice between refractory and ceramic oxides depends on the specific chemical environment and operational temperature, optimizing catalyst performance and lifespan.
Interaction with Active Catalytic Phases
Refractory materials, such as alumina and zirconia, offer robust thermal stability and strong mechanical strength, promoting effective dispersion and anchoring of active catalytic phases, which enhances catalyst durability and performance at high temperatures. Ceramic oxides, including titania and ceria, possess unique redox properties and oxygen storage capacity that facilitate dynamic interactions with active sites, improving catalytic activity and resistance to sintering. The choice between refractory and ceramic oxide supports significantly impacts catalyst efficiency by influencing metal-support interactions, electronic effects, and the overall stability of the active catalytic phases.
Cost and Scalability Considerations
Refractory materials generally offer lower initial costs and better scalability due to their widespread availability and ease of fabrication, making them suitable for large-scale catalyst applications. Ceramic oxides, while more expensive due to complex synthesis and higher purity requirements, provide superior thermal stability and longer operational lifespans, which can reduce long-term replacement costs. Cost-effectiveness must balance upfront investment against durability and performance, with refractory options favored in high-volume production and ceramic oxides preferred for specialized, high-demand catalysts.
Application-Specific Performance
Refractory materials exhibit superior thermal stability and mechanical strength, making them ideal for high-temperature catalytic converters in automotive and industrial applications. Ceramic oxides, such as alumina and zirconia, provide excellent surface area and chemical inertness, enhancing catalyst dispersion and activity in chemical reactors and environmental catalysts. Application-specific performance depends on balancing thermal resilience for harsh conditions with catalytic efficiency for targeted chemical reactions.
Future Trends in Catalyst Support Materials
Future trends in catalyst support materials emphasize the development of advanced ceramic oxides with enhanced thermal stability, higher surface area, and improved resistance to sintering, surpassing traditional refractory supports like alumina and silica. Research highlights the use of mixed metal oxides and doped ceramics to optimize catalytic performance and durability under extreme reaction conditions. Emerging nanostructured ceramic oxides offer tailored porosity and active site dispersion, driving innovations in automotive, chemical, and environmental catalysis applications.
Infographic: Refractory vs Ceramic oxide for Catalyst
 azmater.com
azmater.com