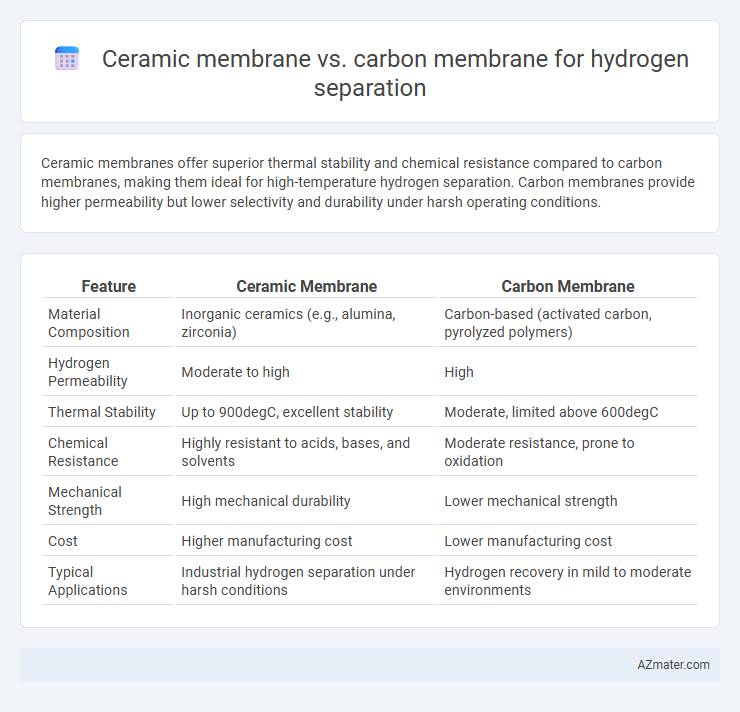
Ceramic membranes offer superior thermal stability and chemical resistance compared to carbon membranes, making them ideal for high-temperature hydrogen separation. Carbon membranes provide higher permeability but lower selectivity and durability under harsh operating conditions.
Table of Comparison
| Feature | Ceramic Membrane | Carbon Membrane |
|---|---|---|
| Material Composition | Inorganic ceramics (e.g., alumina, zirconia) | Carbon-based (activated carbon, pyrolyzed polymers) |
| Hydrogen Permeability | Moderate to high | High |
| Thermal Stability | Up to 900degC, excellent stability | Moderate, limited above 600degC |
| Chemical Resistance | Highly resistant to acids, bases, and solvents | Moderate resistance, prone to oxidation |
| Mechanical Strength | High mechanical durability | Lower mechanical strength |
| Cost | Higher manufacturing cost | Lower manufacturing cost |
| Typical Applications | Industrial hydrogen separation under harsh conditions | Hydrogen recovery in mild to moderate environments |
Introduction to Hydrogen Separation Technologies
Hydrogen separation technologies rely on selective membranes to achieve high purity and efficiency, with ceramic and carbon membranes being prominent options. Ceramic membranes offer exceptional thermal and chemical stability, enabling operation in harsh environments and high temperatures above 400degC. Carbon membranes provide high hydrogen permeability and selectivity, making them suitable for moderate temperature ranges typically between 200degC and 400degC, aligning with specific industrial applications.
Overview of Ceramic Membranes
Ceramic membranes offer superior thermal and chemical stability, making them ideal for high-temperature hydrogen separation processes. Their porous structure enables selective hydrogen permeation while resisting corrosion and fouling in harsh industrial environments. These membranes frequently outperform carbon membranes in terms of durability and operational lifespan during hydrogen purification.
Overview of Carbon Membranes
Carbon membranes for hydrogen separation exhibit high permeability and selective adsorption properties, making them effective for gas mixture separation. Their micro-porous structure allows efficient hydrogen transport while rejecting larger molecules, enhancing purity and flux rates. Compared to ceramic membranes, carbon membranes offer greater chemical stability under harsh operating conditions and lower fabrication costs, supporting scalable industrial applications.
Mechanisms of Hydrogen Separation
Ceramic membranes separate hydrogen through molecular sieving and surface diffusion, leveraging their rigid, thermally stable porous structures that selectively allow hydrogen molecules to pass while blocking larger gases. Carbon membranes rely on adsorption and surface diffusion mechanisms, where hydrogen molecules adsorb onto the carbon surface and diffuse through micropores, benefiting from high surface area but less thermal stability. The pore size distribution and chemical affinity in ceramic membranes provide superior selectivity and durability for hydrogen separation compared to carbon membranes.
Permeability and Selectivity Comparison
Ceramic membranes exhibit higher selectivity for hydrogen separation due to their stable porous structure, effectively blocking larger gas molecules while allowing hydrogen to permeate. Carbon membranes demonstrate superior permeability, benefiting from their unique microporous carbon network that facilitates rapid hydrogen diffusion. The trade-off between permeability and selectivity favors ceramic membranes in applications demanding high purity, whereas carbon membranes suit processes prioritizing flux rates.
Material Stability and Durability
Ceramic membranes exhibit superior material stability and durability compared to carbon membranes when used for hydrogen separation, maintaining structural integrity at high temperatures exceeding 700degC and resisting chemical degradation in harsh environments. Carbon membranes, while effective in selective hydrogen permeation, are prone to oxidation and mechanical wear, limiting their operational lifespan and suitability for long-term industrial applications. The inherent thermal and chemical resilience of ceramic membranes makes them more reliable for continuous hydrogen purification processes under extreme conditions.
Operating Conditions and Performance
Ceramic membranes for hydrogen separation operate effectively at high temperatures of 400-900degC, offering exceptional thermal and chemical stability, which enhances their selectivity and flux rates in harsh industrial environments. Carbon membranes typically function at lower temperatures, around 200-300degC, and exhibit good permeability but lower thermal resilience, making them suitable for less aggressive conditions. Ceramic membranes generally demonstrate higher hydrogen purity and longer operational lifespan under extreme conditions compared to carbon membranes, which can suffer from oxidation and degradation at elevated temperatures.
Cost Analysis and Scalability
Ceramic membranes exhibit higher initial costs due to costly raw materials and complex fabrication processes, but they offer superior durability and chemical resistance, leading to lower replacement expenses over time. Carbon membranes provide a cost-effective alternative with simpler manufacturing methods and lower upfront investment, yet they face challenges in longevity and performance stability under harsh operating conditions. Scalability for ceramic membranes is limited by production complexity and energy-intensive sintering, whereas carbon membranes benefit from easier scale-up and adaptability to various industrial hydrogen separation applications.
Environmental Impact and Sustainability
Ceramic membranes exhibit superior chemical stability and higher temperature resistance, enabling efficient hydrogen separation with minimal environmental degradation and extended lifespan, reducing frequent replacements. Carbon membranes offer lower energy consumption during production but may face challenges in durability and fouling resistance, potentially increasing waste and environmental burden over time. The sustainable choice depends on balancing ceramic membranes' longer operational life and recyclability against carbon membranes' initial lower energy footprint.
Applications and Future Prospects
Ceramic membranes offer high thermal stability and chemical resistance, making them ideal for hydrogen separation in high-temperature industrial processes such as refining and ammonia production. Carbon membranes provide superior permeability and selectivity at lower temperatures, benefiting hydrogen purification in fuel cells and gas processing applications. Future prospects include hybrid membrane systems combining ceramic and carbon materials to enhance efficiency and durability for large-scale hydrogen production and clean energy technologies.
Infographic: Ceramic membrane vs Carbon membrane for Hydrogen separation
 azmater.com
azmater.com