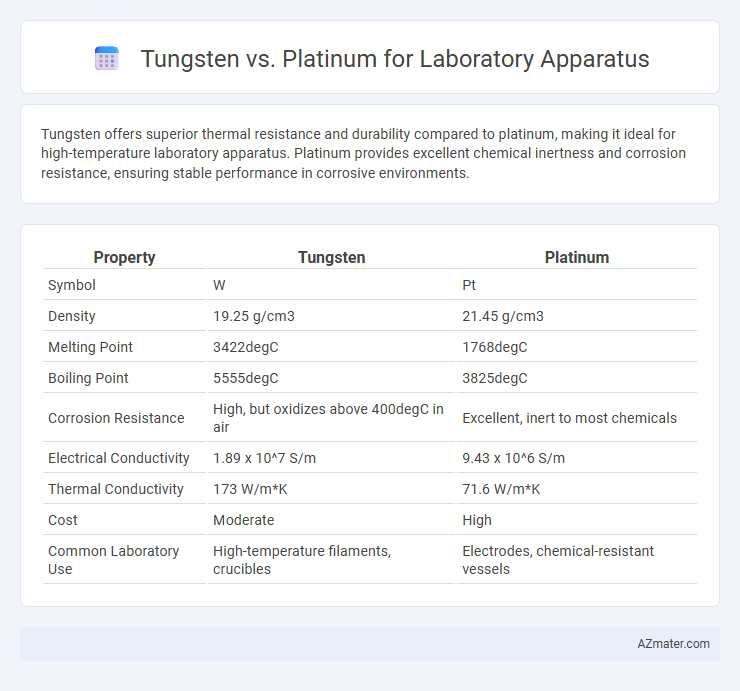
Tungsten offers superior thermal resistance and durability compared to platinum, making it ideal for high-temperature laboratory apparatus. Platinum provides excellent chemical inertness and corrosion resistance, ensuring stable performance in corrosive environments.
Table of Comparison
| Property | Tungsten | Platinum |
|---|---|---|
| Symbol | W | Pt |
| Density | 19.25 g/cm3 | 21.45 g/cm3 |
| Melting Point | 3422degC | 1768degC |
| Boiling Point | 5555degC | 3825degC |
| Corrosion Resistance | High, but oxidizes above 400degC in air | Excellent, inert to most chemicals |
| Electrical Conductivity | 1.89 x 10^7 S/m | 9.43 x 10^6 S/m |
| Thermal Conductivity | 173 W/m*K | 71.6 W/m*K |
| Cost | Moderate | High |
| Common Laboratory Use | High-temperature filaments, crucibles | Electrodes, chemical-resistant vessels |
Introduction: Importance of Material Selection in Laboratory Apparatus
Material selection in laboratory apparatus significantly impacts performance, durability, and safety during experiments. Tungsten and platinum are preferred for their exceptional resistance to high temperatures and chemical corrosion, making them ideal for rigorous laboratory applications. Understanding their distinct thermal conductivity, melting points, and chemical inertness ensures optimized equipment functionality and experiment accuracy.
Overview of Tungsten and Platinum Properties
Tungsten features exceptional hardness, a melting point of 3422degC, and excellent corrosion resistance, making it ideal for high-temperature laboratory apparatus. Platinum offers outstanding chemical inertness, electrical conductivity, and a melting point of 1768degC, ensuring durability in corrosive environments. Both metals provide unique advantages for lab equipment, with tungsten excelling in extreme heat applications and platinum preferred for chemical stability.
Thermal Stability: Tungsten vs Platinum
Tungsten exhibits superior thermal stability with a melting point of 3422degC, significantly higher than platinum's 1768degC, making it ideal for high-temperature laboratory apparatus. Its resistance to thermal deformation ensures consistent performance in extreme heat conditions, whereas platinum, while corrosion-resistant, is more susceptible to melting and warping at elevated temperatures. Tungsten's robustness under thermal stress enhances its durability and reliability in scientific applications requiring sustained high heat.
Chemical Resistance and Reactivity Comparison
Tungsten exhibits exceptional chemical resistance, remaining stable in most acids and alkalis, which makes it ideal for laboratory apparatus exposed to aggressive chemicals. Platinum offers superior chemical inertness, resisting corrosion even in strong oxidizing environments, making it highly suitable for highly reactive or acidic lab conditions. While tungsten is harder and more abrasion-resistant, platinum's lower reactivity ensures minimal contamination in sensitive analytical procedures.
Durability and Longevity in Laboratory Environments
Tungsten exhibits superior durability in laboratory apparatus due to its exceptional hardness and high melting point of 3422degC, making it highly resistant to wear and thermal degradation. Platinum, while chemically inert and corrosion-resistant, has a lower melting point of 1768degC and is softer, which can reduce its longevity under intense mechanical stress. In environments requiring extreme temperature stability and durability, tungsten often outperforms platinum, ensuring extended service life in rigorous laboratory applications.
Cost Analysis: Tungsten vs Platinum
Tungsten offers a significantly lower cost compared to platinum, making it a more budget-friendly option for laboratory apparatus without compromising durability or temperature resistance. Platinum's high price is attributed to its rarity and superior chemical inertness, which can justify the investment for specialized applications requiring exceptional corrosion resistance. Cost analysis reveals tungsten's advantage in large-scale or routine laboratory use, while platinum remains preferable for high-precision or highly corrosive environments where long-term material stability is critical.
Applications Specific to Tungsten Laboratory Apparatus
Tungsten laboratory apparatus excels in high-temperature applications due to its exceptional melting point of 3422degC, making it ideal for use in furnaces, crucibles, and electrodes where heat resistance is critical. Its superior chemical inertness and low vapor pressure ensure durability and stability in reactions involving aggressive chemicals or vacuum conditions. Compared to platinum, tungsten is preferred in scenarios requiring intense thermal stress resistance, such as in spectroscopy, high-temperature synthesis, and materials science research.
Applications Specific to Platinum Laboratory Apparatus
Platinum laboratory apparatus excels in high-temperature applications and chemical resistance, making it ideal for crucibles, electrodes, and catalytic converters used in analytical chemistry. Its exceptional stability and inertness in corrosive environments outperform tungsten, which, despite its high melting point, reacts with certain chemicals and oxidizes at elevated temperatures. Platinum's unparalleled durability in acid digestion, electrochemistry, and gas analysis ensures precise and contamination-free laboratory results.
Maintenance and Handling Considerations
Tungsten laboratory apparatus offers superior resistance to wear and chemical corrosion, making maintenance less frequent compared to platinum, which requires meticulous cleaning to preserve its catalytic properties. Handling tungsten demands careful temperature management due to its high melting point and brittleness, whereas platinum's ductility allows easier manipulation but with increased risk of surface contamination. Both materials benefit from inert atmosphere storage to prevent oxidation and prolong lifespan in sensitive laboratory environments.
Conclusion: Choosing the Ideal Material for Laboratory Needs
Tungsten offers exceptional strength and high melting points ideal for extreme temperature laboratory applications, while platinum provides superior chemical resistance and stability in corrosive environments. Selecting between tungsten and platinum depends on specific lab requirements like temperature tolerance, chemical exposure, and budget considerations. Opting for tungsten ensures durability under thermal stress, whereas platinum guarantees reliability in sensitive chemical analyses.
Infographic: Tungsten vs Platinum for Laboratory Apparatus
 azmater.com
azmater.com