Silver offers superior electrical conductivity and corrosion resistance in battery applications, enhancing efficiency and longevity. Nickel provides cost-effective durability and higher energy density but is more prone to corrosion compared to silver.
Table of Comparison
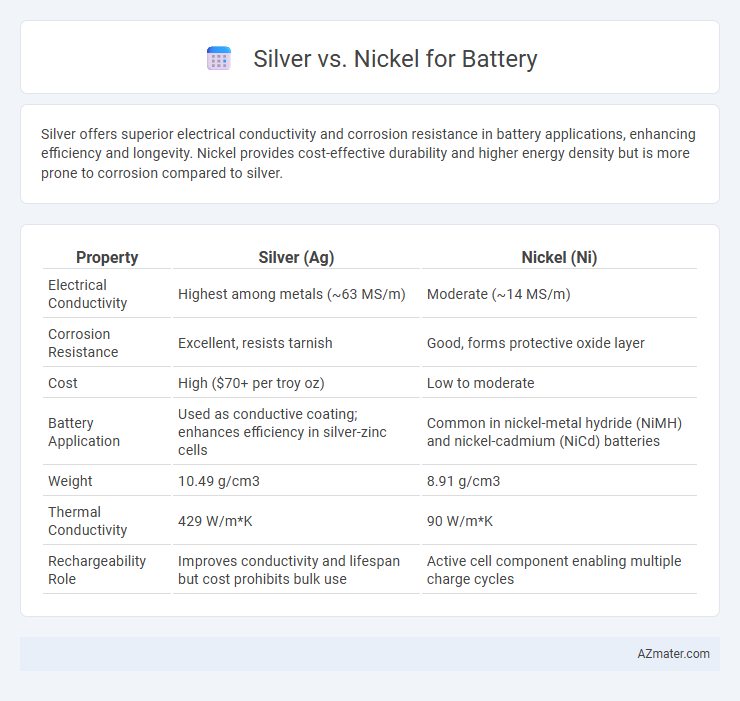
| Property | Silver (Ag) | Nickel (Ni) |
|---|---|---|
| Electrical Conductivity | Highest among metals (~63 MS/m) | Moderate (~14 MS/m) |
| Corrosion Resistance | Excellent, resists tarnish | Good, forms protective oxide layer |
| Cost | High ($70+ per troy oz) | Low to moderate |
| Battery Application | Used as conductive coating; enhances efficiency in silver-zinc cells | Common in nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries |
| Weight | 10.49 g/cm3 | 8.91 g/cm3 |
| Thermal Conductivity | 429 W/m*K | 90 W/m*K |
| Rechargeability Role | Improves conductivity and lifespan but cost prohibits bulk use | Active cell component enabling multiple charge cycles |
Introduction: Silver vs Nickel in Battery Technology
Silver and nickel represent two critical metals in battery technology, each offering distinct electrochemical properties that influence performance and cost. Silver provides superior electrical conductivity and corrosion resistance, enhancing battery efficiency and lifespan, particularly in silver-zinc and silver-cadmium batteries. Nickel, widely used in nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries, balances energy density and durability with affordability, making it a prevalent choice for rechargeable power sources.
Chemical Properties of Silver and Nickel
Silver exhibits excellent electrical conductivity and chemical stability, making it highly resistant to oxidation and corrosion in battery applications. Nickel, with its variable oxidation states and strong catalytic properties, contributes to efficient electrochemical reactions in rechargeable batteries. The distinct chemical properties of silver and nickel influence battery performance, longevity, and charge efficiency.
Energy Density Comparison
Silver-based batteries exhibit significantly higher energy density compared to nickel-based counterparts, primarily due to silver's superior electrochemical properties and higher electrical conductivity. Silver-zinc batteries can achieve energy densities exceeding 130 Wh/kg, whereas nickel-based batteries, such as nickel-cadmium or nickel-metal hydride, typically range between 45 to 80 Wh/kg. The enhanced energy density of silver batteries makes them ideal for applications demanding lightweight power sources with extended operational life.
Conductivity and Efficiency
Silver exhibits the highest electrical conductivity among metals, with a conductivity of approximately 63 x 10^6 S/m, significantly surpassing nickel's conductivity of around 14.3 x 10^6 S/m, which enhances electron flow and battery efficiency. Nickel, however, is commonly used in battery electrodes due to its excellent corrosion resistance and electrochemical properties, despite its lower conductivity. The superior conductivity of silver improves charge transfer rates and reduces internal resistance, potentially increasing battery performance and energy efficiency in high-demand applications.
Cycle Life and Longevity
Silver-based batteries typically exhibit superior cycle life compared to nickel-based counterparts due to silver's higher electrical conductivity and corrosion resistance. Nickel batteries, such as nickel-cadmium (NiCd) or nickel-metal hydride (NiMH), generally offer shorter longevity because of faster capacity degradation and increased memory effect over repeated charge-discharge cycles. Optimizing battery design often favors silver electrodes for applications demanding extended cycle life and long-term reliability.
Cost and Availability
Silver offers superior conductivity but comes at a significantly higher cost compared to nickel, making it less economical for large-scale battery production. Nickel is widely available and cheaper, supporting its widespread use in rechargeable batteries like lithium-ion. The cost-effectiveness and abundance of nickel drive its dominance in battery manufacturing despite silver's performance advantages.
Environmental Impact and Sustainability
Silver batteries offer high conductivity and corrosion resistance, but silver mining generates significant environmental pollutants and depletes finite natural resources. Nickel batteries, widely used in rechargeable cells, have a more established recycling infrastructure, reducing environmental impact and promoting sustainability through material recovery. The sustainability of nickel is enhanced by advancements in extraction methods and increased battery recycling rates, making it a more eco-friendly choice compared to silver in large-scale battery applications.
Applications in Modern Batteries
Silver offers superior electrical conductivity and corrosion resistance, making it ideal for high-performance batteries used in medical devices, military applications, and small-scale energy storage. Nickel's affordability and high energy density are favored in large-scale applications such as electric vehicle batteries and renewable energy storage systems. Both metals enhance battery longevity and efficiency but serve distinct roles based on application-specific requirements.
Safety Considerations
Silver-based batteries exhibit superior thermal stability and lower risk of overheating compared to nickel-based counterparts, reducing potential fire hazards during operation. Nickel batteries, particularly nickel-metal hydride (NiMH), are prone to thermal runaway under abusive conditions, necessitating robust battery management systems for safe use. The inherent chemical stability of silver electrodes contributes to enhanced safety profiles in high-demand energy storage applications.
Future Prospects in Battery Innovation
Silver's superior conductivity and corrosion resistance present significant advantages for future battery technologies, especially in high-performance and flexible energy storage systems. Nickel remains a cornerstone in current lithium-ion battery cathodes due to its high energy density and cost-effectiveness, with ongoing research focused on enhancing its stability and lifespan. Emerging innovations aim to combine silver's conductivity with nickel's energy capacity to develop hybrid batteries that offer improved efficiency and durability for electric vehicles and renewable energy storage.
Infographic: Silver vs Nickel for Battery
 azmater.com
azmater.com