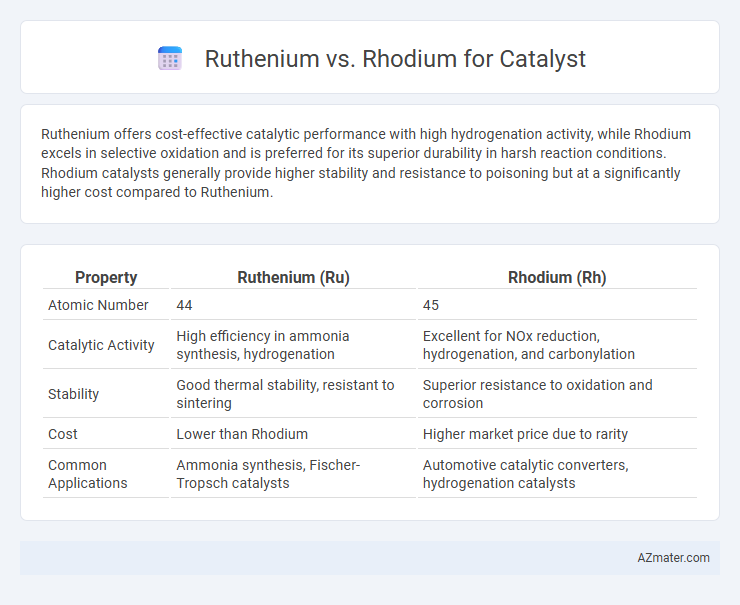
Ruthenium offers cost-effective catalytic performance with high hydrogenation activity, while Rhodium excels in selective oxidation and is preferred for its superior durability in harsh reaction conditions. Rhodium catalysts generally provide higher stability and resistance to poisoning but at a significantly higher cost compared to Ruthenium.
Table of Comparison
| Property | Ruthenium (Ru) | Rhodium (Rh) |
|---|---|---|
| Atomic Number | 44 | 45 |
| Catalytic Activity | High efficiency in ammonia synthesis, hydrogenation | Excellent for NOx reduction, hydrogenation, and carbonylation |
| Stability | Good thermal stability, resistant to sintering | Superior resistance to oxidation and corrosion |
| Cost | Lower than Rhodium | Higher market price due to rarity |
| Common Applications | Ammonia synthesis, Fischer-Tropsch catalysts | Automotive catalytic converters, hydrogenation catalysts |
Introduction to Ruthenium and Rhodium Catalysts
Ruthenium and rhodium catalysts are widely used in chemical reactions due to their exceptional catalytic properties in hydrogenation, oxidation, and carbon-carbon bond formation. Ruthenium catalysts excel in ammonia synthesis and olefin metathesis, displaying high activity and selectivity under mild conditions. Rhodium catalysts are renowned for their efficiency in hydroformylation and asymmetric hydrogenation processes, making them crucial in fine chemical and pharmaceutical production.
Chemical Properties and Reactivity
Ruthenium exhibits versatile catalytic activity due to its ability to adopt multiple oxidation states, primarily ranging from +2 to +8, enabling efficient hydrogenation, oxidation, and ammonia synthesis reactions. Rhodium is highly valued for its exceptional catalytic reactivity in processes such as catalytic converters and hydroformylation, owing to its stable +3 oxidation state and strong resistance to oxidation. While ruthenium offers greater flexibility in oxidation-state-driven reactivity, rhodium's superior resistance to poisoning and high selectivity makes it ideal for precision catalytic applications.
Common Industrial Applications
Ruthenium and rhodium are widely used as catalysts in industrial processes due to their exceptional catalytic properties. Ruthenium is preferred in ammonia synthesis and Fischer-Tropsch processes, enhancing efficiency and selectivity in fertilizer production and hydrocarbon conversion. Rhodium dominates automotive catalytic converters and hydrogenation reactions, effectively reducing emissions and facilitating fine chemical synthesis.
Catalytic Efficiency: Ruthenium vs Rhodium
Rhodium exhibits superior catalytic efficiency compared to ruthenium, particularly in hydrogenation and carbonylation reactions, due to its higher selectivity and turnover frequency. Ruthenium, while effective in ammonia synthesis and Fischer-Tropsch catalysis, generally demonstrates lower activity and requires harsher conditions. The choice between ruthenium and rhodium catalysts often depends on the specific reaction mechanism and desired product yield.
Selectivity in Chemical Reactions
Ruthenium and rhodium catalysts exhibit distinct selectivity profiles in chemical reactions, with ruthenium often favoring hydrogenation and ammonia synthesis due to its high activity on nitrogen-containing substrates. Rhodium catalysts demonstrate superior selectivity in hydroformylation and carbonylation processes, enabling precise control over product distribution and minimizing side reactions. The choice between ruthenium and rhodium catalysts hinges on the desired reaction pathway and target molecule specificity, impacting yield and purity in industrial applications.
Cost and Availability Comparison
Ruthenium and rhodium are both precious metals widely used as catalysts in automotive and chemical industries, but rhodium is significantly more expensive due to its rarity and high demand in emission control technologies. Ruthenium, although less costly, is more abundant in the Earth's crust and offers a cost-effective alternative for certain catalytic applications, especially in ammonia synthesis and hydrogenation reactions. The cost disparity and supply constraints make rhodium a premium choice for catalytic converters, while ruthenium's availability provides advantages for large-scale industrial use where budget constraints are critical.
Environmental Impact and Sustainability
Ruthenium catalysts exhibit lower toxicity and greater abundance compared to rhodium, making them a more environmentally sustainable choice for catalytic applications. Rhodium, while highly effective in catalytic converters for reducing vehicle emissions, is rarer and involves more energy-intensive mining processes, contributing to a higher environmental footprint. The preference for ruthenium supports sustainable development goals by minimizing resource depletion and reducing hazardous waste in catalytic systems.
Recent Advances in Catalyst Technology
Ruthenium and Rhodium have emerged as pivotal catalysts in recent advancements, with Ruthenium showing superior efficiency in ammonia synthesis and hydrogenation due to its unique electronic properties. Rhodium excels in automotive catalytic converters and selective hydrogenation, benefiting from enhanced durability and resistance to poisoning under harsh conditions. Innovations in nano-structuring and alloy compositions have significantly improved the catalytic activity and stability of both metals, positioning them as critical components in sustainable chemical processes and green energy applications.
Challenges and Limitations
Ruthenium catalysts often face challenges related to their sensitivity to air and moisture, which can lead to deactivation and reduced catalytic efficiency. Rhodium, although more stable under oxidative conditions, presents limitations due to its high cost and scarcity, impacting its widespread industrial application. Both metals require careful handling and optimization to overcome issues of selectivity and long-term stability in catalytic processes.
Future Perspectives in Catalyst Development
Ruthenium and rhodium, both platinum-group metals, exhibit unique catalytic properties essential for future catalyst development in sustainable chemical processes. Ruthenium's versatility in hydrogenation and ammonia synthesis positions it as a key candidate for green energy applications, while rhodium's exceptional activity and selectivity in automotive catalytic converters drive innovation in emission control technologies. Advances in nanostructuring and alloy design promise enhanced efficiency and durability of ruthenium- and rhodium-based catalysts, paving the way for more cost-effective, environmentally friendly catalytic solutions.
Infographic: Ruthenium vs Rhodium for Catalyst
 azmater.com
azmater.com