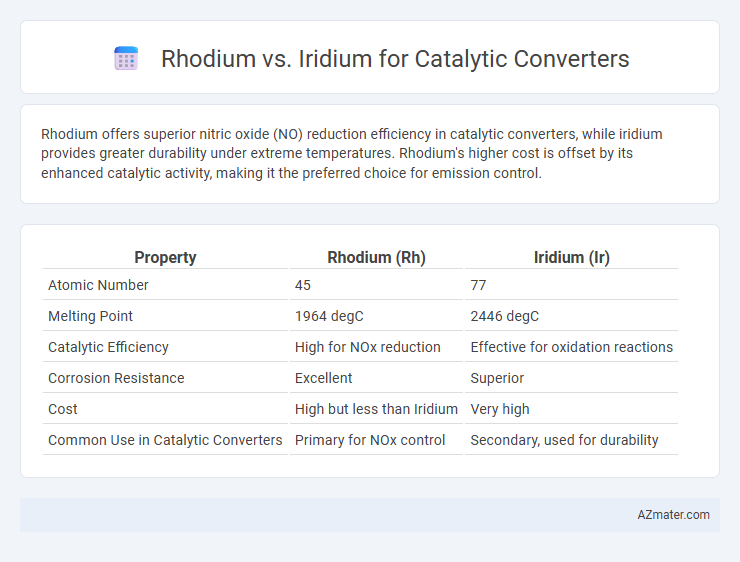
Rhodium offers superior nitric oxide (NO) reduction efficiency in catalytic converters, while iridium provides greater durability under extreme temperatures. Rhodium's higher cost is offset by its enhanced catalytic activity, making it the preferred choice for emission control.
Table of Comparison
| Property | Rhodium (Rh) | Iridium (Ir) |
|---|---|---|
| Atomic Number | 45 | 77 |
| Melting Point | 1964 degC | 2446 degC |
| Catalytic Efficiency | High for NOx reduction | Effective for oxidation reactions |
| Corrosion Resistance | Excellent | Superior |
| Cost | High but less than Iridium | Very high |
| Common Use in Catalytic Converters | Primary for NOx control | Secondary, used for durability |
Introduction to Catalytic Converters
Catalytic converters utilize rhodium and iridium as critical catalytic metals to reduce harmful emissions from internal combustion engines, transforming pollutants like nitrogen oxides into less harmful nitrogen and oxygen. Rhodium exhibits exceptional efficiency in NOx reduction, while iridium contributes to durability and resistance under high-temperature conditions. The combination of these platinum-group metals ensures optimal performance and longevity in automotive exhaust systems.
Overview of Precious Metals in Catalysis
Rhodium and iridium are both platinum-group metals extensively used in catalytic converters due to their exceptional catalytic properties and resistance to high temperatures. Rhodium is highly effective at reducing nitrogen oxides (NOx) emissions, while iridium contributes durability and oxidation resistance in harsh exhaust environments. The synergy of these precious metals enhances overall catalytic performance, crucial for meeting stringent emission standards.
Chemical Properties: Rhodium vs Iridium
Rhodium exhibits exceptional catalytic properties due to its high resistance to oxidation and ability to adsorb nitrogen oxides, making it highly effective in catalytic converters for reducing NOx emissions. Iridium, though chemically similar as a platinum-group metal, shows greater chemical stability and higher melting point but lower catalytic activity for NOx reduction compared to rhodium. The choice between rhodium and iridium in catalytic converters hinges on rhodium's superior activity in facilitating nitrogen oxide conversion under high-temperature exhaust conditions.
Catalytic Efficiency in Emission Control
Rhodium exhibits superior catalytic efficiency in converting nitrogen oxides (NOx) into harmless nitrogen and oxygen, making it a critical component in catalytic converters for emission control. Iridium, while effective as a catalyst, shows lower selectivity and activity for NOx reduction compared to rhodium, which results in less efficient emission control. The high catalytic activity and thermal stability of rhodium ensure optimal performance in reducing harmful exhaust gases, thereby enhancing overall catalytic converter efficiency.
Durability and Thermal Stability Comparison
Rhodium exhibits superior catalytic durability compared to iridium, maintaining efficiency under prolonged exposure to high exhaust temperatures commonly exceeding 900degC. Iridium demonstrates exceptional thermal stability with a melting point of 2,446degC, surpassing rhodium's 1,964degC, allowing it to resist sintering and structural degradation at extreme conditions. However, rhodium's enhanced resistance to thermal aging and poisoning by sulfur compounds often results in longer-lasting catalytic performance in automotive catalytic converters.
Cost and Market Availability Analysis
Rhodium commands a significantly higher price per ounce compared to iridium, primarily due to its critical role in automotive catalytic converters and limited global supply. Iridium offers more stable market availability with slightly lower costs, making it a viable alternative in catalytic applications despite its lower catalytic efficiency. Fluctuations in rhodium prices, driven by mining constraints and automotive demand, often influence manufacturers to balance performance needs with cost-effectiveness by incorporating iridium.
Environmental Impact of Mining and Processing
Rhodium and iridium, both precious metals used in catalytic converters, have distinct environmental impacts related to their mining and processing. Rhodium mining often involves extensive platinum group metal (PGM) extraction in South Africa, leading to significant habitat disruption, water pollution, and high energy consumption. Iridium mining is typically a byproduct of nickel and platinum extraction, resulting in comparatively lower direct environmental degradation but still contributes to deforestation and soil contamination in mining regions.
Technological Trends in Converter Design
Rhodium and iridium are critical noble metals in catalytic converter technology, with rhodium primarily used for NOx reduction and iridium gaining attention for its stability in extreme conditions. Recent advancements focus on enhancing rhodium's efficiency with lower amounts through nano-structured catalysts and improving iridium's durability to withstand higher temperatures in next-generation converters. Emerging designs integrate these metals into multi-functional catalysts to optimize emission control while reducing precious metal load and overall environmental impact.
Industry Preferences and Current Applications
Rhodium is favored in catalytic converters for its exceptional ability to reduce nitrogen oxides (NOx), making it the primary choice in automotive pollution control. Iridium, though less common, is valued in high-performance or specialized catalytic converters due to its robustness and resistance to sintering at extreme temperatures. Industry preferences lean heavily towards rhodium for mass-produced vehicles, while iridium finds niche applications where durability under severe conditions is critical.
Future Prospects in Catalyst Innovation
Rhodium and iridium, both platinum-group metals, continue to drive advancements in catalytic converter efficiency and durability, with rhodium excelling in NOx reduction and iridium emerging in high-temperature resistance applications. Future catalyst innovation focuses on maximizing precious metal utilization through nano-structured alloys and developing hybrid catalysts that integrate rhodium and iridium to enhance overall emission control performance. Research prioritizes cost-effective synthesis methods and the recyclability of these metals to meet stricter environmental regulations and growing demand for clean automotive technologies.
Infographic: Rhodium vs Iridium for Catalytic Converter
 azmater.com
azmater.com