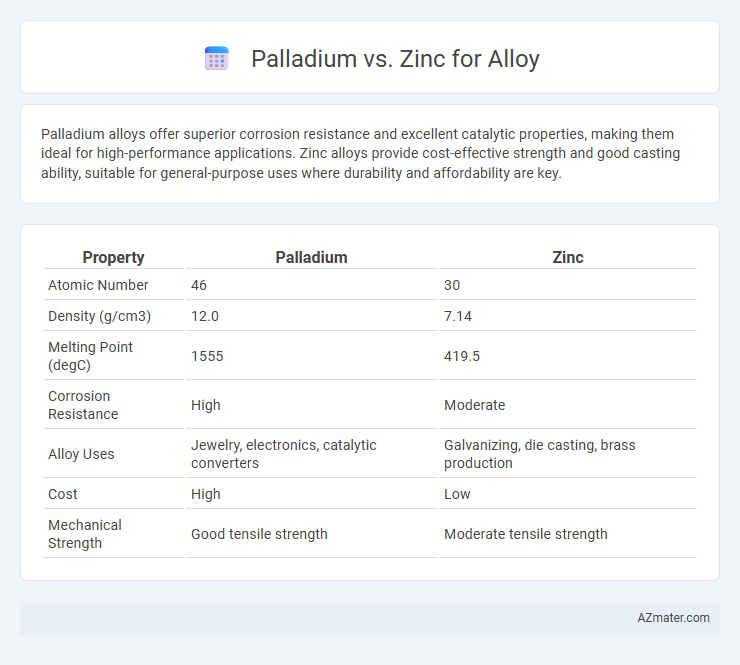
Palladium alloys offer superior corrosion resistance and excellent catalytic properties, making them ideal for high-performance applications. Zinc alloys provide cost-effective strength and good casting ability, suitable for general-purpose uses where durability and affordability are key.
Table of Comparison
| Property | Palladium | Zinc |
|---|---|---|
| Atomic Number | 46 | 30 |
| Density (g/cm3) | 12.0 | 7.14 |
| Melting Point (degC) | 1555 | 419.5 |
| Corrosion Resistance | High | Moderate |
| Alloy Uses | Jewelry, electronics, catalytic converters | Galvanizing, die casting, brass production |
| Cost | High | Low |
| Mechanical Strength | Good tensile strength | Moderate tensile strength |
Introduction to Palladium and Zinc Alloys
Palladium and zinc alloys are widely used in various industries due to their distinct properties and applications. Palladium alloys are prized for their corrosion resistance, durability, and excellent electrical conductivity, making them ideal for electronics, jewelry, and dental materials. Zinc alloys offer superior castability, strength, and cost-effectiveness, which suits automotive parts, die-casting, and construction components.
Chemical Properties: Palladium vs Zinc
Palladium exhibits exceptional chemical stability and resistance to oxidation, making it highly durable in alloy applications, whereas zinc is more reactive and prone to corrosion under certain conditions. Palladium's noble metal characteristics contribute to its excellent catalytic properties and corrosion resistance, unlike zinc, which acts as a sacrificial anode to protect other metals from rust. The significant difference in electronegativity and standard electrode potentials between palladium (+0.951 V) and zinc (-0.763 V) influences their distinct chemical behaviors in alloy formation and performance.
Physical Characteristics Comparison
Palladium exhibits a lustrous silvery-white appearance with excellent corrosion resistance and a density of 12.02 g/cm3, making it a heavy yet durable metal for alloys. Zinc is a bluish-white metal with a lower density of 7.14 g/cm3 and moderate corrosion resistance, offering lighter-weight alloy applications. The melting point of palladium is significantly higher at 1,554degC compared to zinc's 420degC, influencing their usability in high-temperature alloy processes.
Alloy Formation: Methods and Processes
Palladium and zinc differ significantly in alloy formation due to their distinct chemical properties and melting points; palladium alloys commonly form through melting and casting processes at high temperatures, often involving platinum group metals for enhanced corrosion resistance. Zinc alloys typically use low-temperature processes like die casting or sand casting, benefiting from zinc's lower melting point and excellent fluidity to produce complex shapes. Both metals undergo processes such as thermal treatment and mechanical working to optimize alloy microstructure, enhancing properties like strength, ductility, and resistance to wear.
Corrosion Resistance in Palladium and Zinc Alloys
Palladium alloys exhibit superior corrosion resistance due to their inert nature and high resistance to oxidation, making them ideal for jewelry and dental applications exposed to harsh environments. Zinc alloys, while offering affordability and strength, are more prone to corrosion, often requiring protective coatings to prevent degradation in moist or acidic conditions. The inherent corrosion resistance of palladium significantly extends the lifespan and maintains the aesthetic quality of alloys compared to zinc-based alternatives.
Cost and Availability of Both Metals
Palladium, a rare and precious metal, commands a significantly higher cost compared to zinc due to its scarcity and extensive use in catalytic converters and electronics. Zinc is abundant, with widespread geographic availability, resulting in a lower price and easier procurement for alloy manufacturing. Enterprises seeking affordable and readily available alloy components typically prefer zinc, while palladium alloys are chosen for high-performance applications despite their premium cost.
Applications in Industry and Technology
Palladium alloys excel in electronics and automotive industries due to their superior corrosion resistance and excellent conductivity, making them ideal for catalytic converters and electronic connectors. Zinc alloys are widely used in die casting and construction, offering cost-effective corrosion protection and high strength for automotive parts, hardware, and marine applications. Both metals serve distinct industrial needs, with palladium favored in high-tech and precision environments and zinc preferred for durability and economic manufacturing.
Environmental Impact of Palladium and Zinc Alloys
Palladium alloys generally exhibit lower environmental impact compared to traditional zinc alloys due to palladium's exceptional corrosion resistance, which reduces the need for frequent replacements and minimizes metal waste. Zinc alloys, while abundant and less costly, often involve more intensive mining and processing activities that contribute to higher energy consumption and greater ecological disruption. Lifecycle assessments highlight palladium's potential for improved sustainability in specialty applications despite its higher initial extraction footprint.
Suitability for Jewelry and Decorative Uses
Palladium offers excellent suitability for jewelry and decorative uses due to its naturally white luster, resistance to tarnish, and hypoallergenic properties, making it ideal for fine pieces and everyday wear. Zinc, while commonly used as a base metal in alloys such as brass, lacks the durability and aesthetic appeal of palladium, as it is prone to corrosion and discoloration over time. The superior hardness and corrosion resistance of palladium alloys provide enhanced longevity and a premium finish, making them preferable for high-quality decorative applications.
Choosing the Right Alloy: Palladium vs Zinc
Palladium offers superior corrosion resistance, ductility, and hypoallergenic properties, making it ideal for high-quality jewelry and electronic components. Zinc alloys provide cost-effective strength and good casting properties, suited for automotive parts and die-casting applications. Selecting the right alloy depends on application requirements; prioritize palladium for durability and biocompatibility, while zinc is preferred for affordability and ease of manufacturing.
Infographic: Palladium vs Zinc for Alloy
 azmater.com
azmater.com