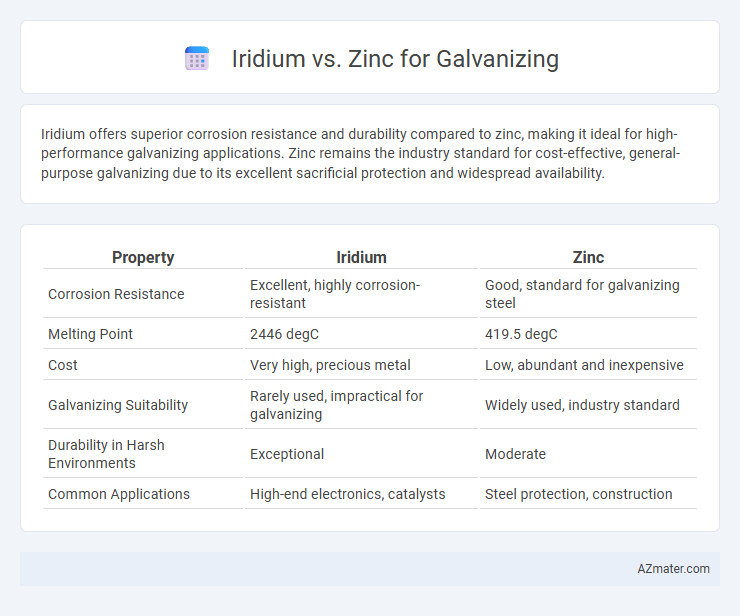
Iridium offers superior corrosion resistance and durability compared to zinc, making it ideal for high-performance galvanizing applications. Zinc remains the industry standard for cost-effective, general-purpose galvanizing due to its excellent sacrificial protection and widespread availability.
Table of Comparison
| Property | Iridium | Zinc |
|---|---|---|
| Corrosion Resistance | Excellent, highly corrosion-resistant | Good, standard for galvanizing steel |
| Melting Point | 2446 degC | 419.5 degC |
| Cost | Very high, precious metal | Low, abundant and inexpensive |
| Galvanizing Suitability | Rarely used, impractical for galvanizing | Widely used, industry standard |
| Durability in Harsh Environments | Exceptional | Moderate |
| Common Applications | High-end electronics, catalysts | Steel protection, construction |
Introduction to Galvanizing: Purpose and Processes
Galvanizing commonly employs zinc as a protective coating to prevent steel corrosion through a process called hot-dip galvanizing, where steel is immersed in molten zinc to form a durable, corrosion-resistant layer. Iridium, although more corrosion-resistant and noble, is rarely used for galvanizing due to its high cost and limited availability, making zinc the practical choice for large-scale industrial applications. The primary purpose of galvanizing is to extend the lifespan of steel structures by providing sacrificial protection, with zinc offering an optimal balance between performance, cost, and scalability in the galvanizing industry.
Overview of Iridium and Zinc as Coating Materials
Iridium and zinc serve distinct roles as coating materials in galvanizing, with zinc being the predominant choice due to its excellent corrosion resistance and cost-effectiveness. Zinc acts as a sacrificial anode, protecting steel by forming a stable oxide layer that prevents rust, whereas iridium, a rare and highly durable platinum-group metal, offers superior hardness and chemical stability but is rarely used due to its high cost. The selection between iridium and zinc hinges on application requirements, balancing zinc's widespread industrial use against iridium's niche advantages in extreme environments.
Chemical Properties: Iridium vs Zinc
Iridium exhibits exceptional chemical stability and resistance to corrosion due to its high electronegativity and noble metal characteristics, making it nearly inert in harsh environments. Zinc, on the other hand, is highly reactive and serves as a sacrificial anode in galvanizing by readily oxidizing to protect steel from rust through the formation of a stable zinc oxide layer. The contrasting chemical properties highlight iridium's inertness versus zinc's sacrificial corrosion resistance, which is crucial for effective galvanizing applications.
Corrosion Resistance: Comparative Performance
Iridium offers superior corrosion resistance compared to zinc due to its exceptional stability and resistance to oxidation in harsh environments. Zinc provides effective sacrificial protection through galvanic action, but it corrodes faster under acidic or marine conditions. For long-term applications requiring minimal maintenance and high durability, iridium-coated surfaces outperform zinc galvanizing in preventing rust and degradation.
Durability and Longevity in Galvanized Coatings
Iridium offers exceptional durability in galvanized coatings due to its high resistance to corrosion and oxidation, extending the lifespan of protective layers significantly. Zinc remains the standard choice for galvanizing with effective corrosion protection through sacrificial anode action, but it generally has a shorter service life compared to iridium-based coatings. Combining iridium with zinc can enhance coating longevity by providing both sacrificial protection and increased resistance to environmental degradation.
Cost Analysis: Iridium vs Zinc for Industrial Use
Iridium is significantly more expensive than zinc, with prices per kilogram reaching thousands of dollars compared to zinc's approximate cost of a few dollars per kilogram, making zinc the more cost-effective choice for large-scale galvanizing operations. Zinc's affordability and widespread availability support its dominance in industrial applications for corrosion protection of steel, whereas iridium's high cost limits its use to specialized applications requiring exceptional durability. The substantial price disparity directly impacts industrial budgeting and scalability, favoring zinc for most galvanizing projects where cost-efficiency is critical.
Environmental Impact and Sustainability Considerations
Iridium is rarely used in galvanizing due to its scarcity and high environmental extraction costs, whereas zinc remains the dominant choice because of its abundant availability and lower ecological footprint. Zinc galvanizing enables corrosion protection with recyclable materials, supporting circular economy principles and minimizing waste. Life cycle assessments demonstrate that zinc coatings contribute less to carbon emissions and resource depletion compared to precious metals like iridium.
Application Suitability: Industries and Use Cases
Zinc is widely used in galvanizing for automotive, construction, and infrastructure industries due to its excellent corrosion resistance and cost-effectiveness. Iridium, though rare and expensive, is suitable for specialized high-performance applications such as aerospace and electronics where superior durability and resistance to harsh environments are critical. Choosing between iridium and zinc depends on the specific industry requirements, with zinc dominating mass-market protective coatings and iridium reserved for niche, high-value use cases.
Maintenance and Life-Cycle Requirements
Iridium coatings offer exceptional corrosion resistance with minimal maintenance due to their inert properties, extending the life-cycle of galvanized structures significantly beyond traditional zinc coatings. Zinc galvanizing, while cost-effective and widely used, requires periodic inspection and touch-ups to prevent corrosion and maintain protective efficacy, especially in harsh environments. The choice between iridium and zinc for galvanizing depends on balancing upfront costs against long-term maintenance savings and durability needs.
Conclusions and Future Trends in Galvanizing Materials
Iridium's exceptional corrosion resistance and catalytic properties position it as a potential premium alternative to zinc in specialized galvanizing applications where durability is critical, despite its higher cost. Zinc remains the industry standard due to its excellent sacrificial protection, cost-effectiveness, and abundance, supporting continued dominance in widespread galvanizing use. Emerging trends point towards hybrid coatings combining zinc with trace elements like iridium to enhance performance while balancing economic viability, alongside advances in nano-coatings and environmentally friendly materials shaping the future of corrosion protection.
Infographic: Iridium vs Zinc for Galvanizing
 azmater.com
azmater.com