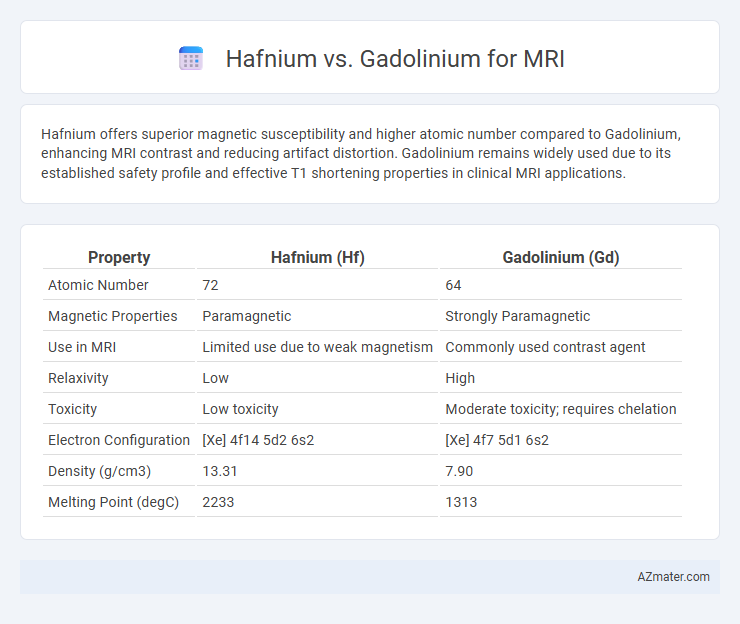
Hafnium offers superior magnetic susceptibility and higher atomic number compared to Gadolinium, enhancing MRI contrast and reducing artifact distortion. Gadolinium remains widely used due to its established safety profile and effective T1 shortening properties in clinical MRI applications.
Table of Comparison
| Property | Hafnium (Hf) | Gadolinium (Gd) |
|---|---|---|
| Atomic Number | 72 | 64 |
| Magnetic Properties | Paramagnetic | Strongly Paramagnetic |
| Use in MRI | Limited use due to weak magnetism | Commonly used contrast agent |
| Relaxivity | Low | High |
| Toxicity | Low toxicity | Moderate toxicity; requires chelation |
| Electron Configuration | [Xe] 4f14 5d2 6s2 | [Xe] 4f7 5d1 6s2 |
| Density (g/cm3) | 13.31 | 7.90 |
| Melting Point (degC) | 2233 | 1313 |
Introduction to MRI Contrast Agents
Hafnium and Gadolinium are elements investigated for use as MRI contrast agents due to their paramagnetic properties, which enhance the relaxation rates of water protons and improve image clarity. Gadolinium-based agents are widely used because of their strong T1 shortening effects, but concerns over toxicity have prompted research into alternatives like Hafnium, which offers promising biocompatibility and potential for reduced side effects. Optimization of these agents involves balancing relaxivity, stability, and safety to achieve precise tissue differentiation in clinical MRI applications.
Overview of Hafnium-Based Contrast Agents
Hafnium-based contrast agents offer promising advantages in MRI due to their high atomic number, which enhances X-ray attenuation and provides improved image contrast compared to traditional gadolinium agents. These agents exhibit lower toxicity and reduced risk of nephrogenic systemic fibrosis, addressing significant safety concerns associated with gadolinium. Ongoing research highlights hafnium's potential for multifunctional imaging and targeted delivery, making it a valuable alternative in the development of next-generation MRI contrast materials.
Gadolinium: The Gold Standard in MRI
Gadolinium remains the gold standard in MRI contrast agents due to its strong paramagnetic properties, which significantly enhance image clarity and diagnostic accuracy. Compared to hafnium, gadolinium chelates exhibit excellent relaxation times, improving the visualization of soft tissues and vascular structures. Concerns over gadolinium retention have led to the development of safer formulations, but its efficacy and widespread clinical use continue to dominate MRI applications.
Chemical Properties of Hafnium and Gadolinium
Hafnium, a transition metal with atomic number 72, exhibits excellent chemical stability and a high melting point of 2233degC, which contributes to its resistance against corrosion and oxidation in MRI environments. Gadolinium, atomic number 64, is a lanthanide known for its seven unpaired 4f electrons, providing strong paramagnetic properties essential for enhancing MRI contrast. The contrasting oxidation states--Hafnium typically at +4 and Gadolinium primarily at +3--impact their coordination chemistry and suitability as MRI agents.
Mechanisms of MRI Signal Enhancement
Hafnium and gadolinium differ significantly in their mechanisms of MRI signal enhancement due to their unique electronic configurations and magnetic properties. Gadolinium, with its seven unpaired electrons in the 4f orbital, is highly paramagnetic, efficiently shortening T1 relaxation times and thus enhancing signal intensity in T1-weighted images. Hafnium, possessing fewer unpaired electrons and different crystallographic behaviors, offers alternative contrast mechanisms primarily through T2* effects and susceptibility variations, making it a potential candidate for specialized imaging applications.
Comparative Safety Profiles
Hafnium and gadolinium differ significantly in MRI safety profiles, with gadolinium-based contrast agents linked to nephrogenic systemic fibrosis (NSF) in patients with renal impairment, while hafnium nanoparticles exhibit lower toxicity and improved biocompatibility. Studies indicate hafnium's reduced risk of metal ion release mitigates adverse reactions common in gadolinium use. Consequently, hafnium presents a safer alternative for patients requiring MRI contrast, especially those with compromised kidney function.
Clinical Efficacy in Diagnostic Imaging
Hafnium-based contrast agents exhibit superior clinical efficacy in MRI due to their higher atomic number, leading to enhanced X-ray attenuation and improved image resolution compared to gadolinium agents. Gadolinium, widely used in diagnostic imaging, poses risks of nephrogenic systemic fibrosis in patients with renal impairment, whereas hafnium offers a safer profile with lower toxicity. Recent studies demonstrate that hafnium-enhanced MRI provides clearer tissue differentiation and faster contrast uptake, making it a promising alternative for precise diagnostic imaging.
Potential Risks and Side Effects
Hafnium-based contrast agents in MRI show promising biocompatibility with lower toxicity risks compared to traditional gadolinium-based agents, which have been linked to nephrogenic systemic fibrosis and gadolinium deposition in the brain. Gadolinium's potential for bioaccumulation raises concerns, especially in patients with kidney impairment, prompting ongoing research into safer alternatives like hafnium nanoparticles. While hafnium's long-term side effects remain under investigation, initial studies suggest reduced allergic reactions and improved safety profiles in MRI diagnostics.
Cost and Accessibility Considerations
Hafnium-based contrast agents for MRI are generally more expensive due to limited availability and complex synthesis processes, impacting accessibility in low-resource settings. Gadolinium agents are widely used with established production methods, making them more cost-effective and readily available worldwide. The growing concerns about gadolinium retention have prompted research into hafnium alternatives, but cost remains a significant barrier for widespread clinical adoption.
Future Prospects and Research Directions
Hafnium-based contrast agents exhibit promising potential due to their superior X-ray attenuation and biocompatibility, positioning them as next-generation candidates for multimodal imaging, including MRI. Current research emphasizes functionalizing hafnium nanoparticles to enhance magnetic resonance signal amplification and targeted delivery, potentially surpassing gadolinium's limitations such as toxicity and accumulation risks. Emerging studies on hafnium complexes aim to optimize relaxivity parameters and investigate synergistic effects with other imaging modalities, driving innovation in safer and more effective MRI contrast agents.
Infographic: Hafnium vs Gadolinium for MRI
 azmater.com
azmater.com