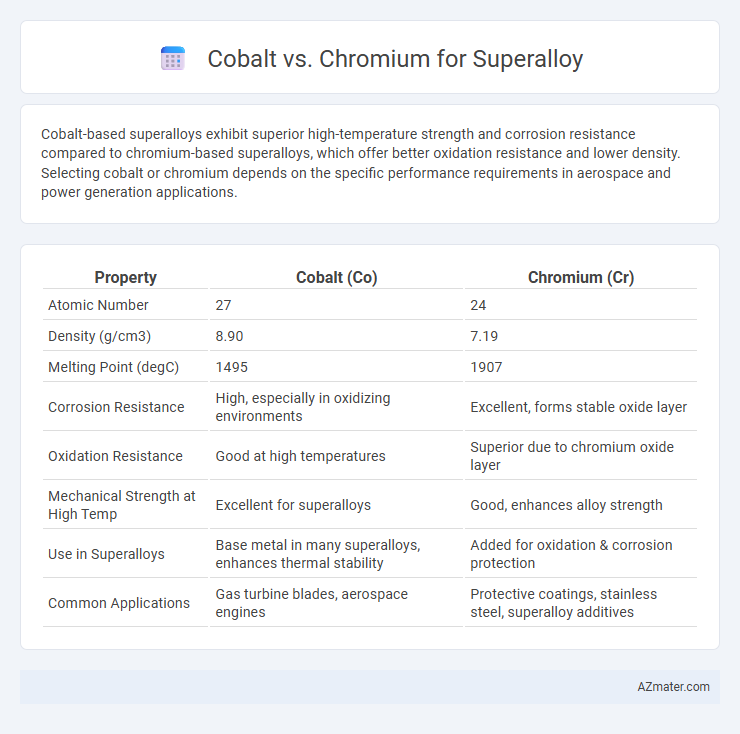
Cobalt-based superalloys exhibit superior high-temperature strength and corrosion resistance compared to chromium-based superalloys, which offer better oxidation resistance and lower density. Selecting cobalt or chromium depends on the specific performance requirements in aerospace and power generation applications.
Table of Comparison
| Property | Cobalt (Co) | Chromium (Cr) |
|---|---|---|
| Atomic Number | 27 | 24 |
| Density (g/cm3) | 8.90 | 7.19 |
| Melting Point (degC) | 1495 | 1907 |
| Corrosion Resistance | High, especially in oxidizing environments | Excellent, forms stable oxide layer |
| Oxidation Resistance | Good at high temperatures | Superior due to chromium oxide layer |
| Mechanical Strength at High Temp | Excellent for superalloys | Good, enhances alloy strength |
| Use in Superalloys | Base metal in many superalloys, enhances thermal stability | Added for oxidation & corrosion protection |
| Common Applications | Gas turbine blades, aerospace engines | Protective coatings, stainless steel, superalloy additives |
Introduction to Superalloys: The Role of Cobalt and Chromium
Superalloys are high-performance materials designed for extreme environments, where Cobalt and Chromium play critical roles. Cobalt provides excellent high-temperature strength and corrosion resistance essential in aerospace and power generation applications. Chromium enhances oxidation resistance and structural stability, making it vital for maintaining superalloy integrity under aggressive operating conditions.
Chemical and Physical Properties Comparison
Cobalt-based superalloys exhibit higher melting points around 1495degC and superior corrosion resistance compared to chromium-based alloys, which typically melt near 1907degC but offer enhanced oxidation resistance. Chemically, cobalt provides excellent stability in high-temperature, oxidative environments due to its ability to form protective oxide layers, whereas chromium contributes primarily to hardness and wear resistance through strong carbide formation. Physically, cobalt superalloys feature greater tensile strength and creep resistance at elevated temperatures, making them ideal for aerospace turbine components, while chromium alloys are better suited for applications requiring enhanced surface hardness and abrasion resistance.
Cobalt in Superalloy Applications
Cobalt-based superalloys exhibit superior high-temperature strength and oxidation resistance compared to chromium-based options, making them ideal for aerospace turbine blades and gas turbine components. The presence of cobalt enhances hot corrosion resistance and mechanical stability under thermal cycling, critical for power generation and aviation industries. These alloys maintain structural integrity at temperatures exceeding 1000degC, outperforming chromium-containing superalloys in extreme operational environments.
Chromium’s Functions and Advantages in Superalloys
Chromium in superalloys primarily enhances oxidation and corrosion resistance by forming a stable, protective oxide layer, significantly improving high-temperature performance in gas turbine engines. Its ability to maintain structural stability and resist scaling under extreme conditions contributes to increased durability and lifespan of critical components. Compared to cobalt, chromium offers superior oxidation resistance, crucial for maintaining strength and integrity in aggressive thermal environments.
Mechanical Strength: Cobalt vs. Chromium Alloys
Cobalt-based superalloys exhibit superior mechanical strength and excellent high-temperature creep resistance compared to chromium alloys, making them ideal for turbine blades and jet engines. Chromium alloys offer good oxidation resistance but lack the high-temperature mechanical robustness found in cobalt superalloys. The enhanced g' phase strengthening in cobalt alloys significantly improves their hardness and durability under extreme thermal and mechanical stress.
Corrosion and Oxidation Resistance
Cobalt-based superalloys exhibit superior corrosion resistance due to their stable oxide scales primarily composed of chromium oxide and spinel phases, enhancing performance in high-temperature oxidative environments. Chromium boosts oxidation resistance by forming a continuous, protective chromium oxide layer but presents limited corrosion resistance compared to cobalt alloys under aggressive conditions such as acidic or chloride-rich environments. In superalloys, cobalt alloys excel in maintaining structural integrity during prolonged exposure to corrosive atmospheres, while chromium additions enhance protective scale adherence but require careful composition control to prevent scale spallation.
High-Temperature Performance
Cobalt-based superalloys exhibit superior high-temperature strength and oxidation resistance compared to chromium-based alloys, making them ideal for turbine blades and jet engine components operating above 900degC. Chromium enhances corrosion resistance and forms stable oxide scales but lacks the elevated temperature creep resistance and thermal stability that cobalt alloys provide. The superior phase stability and gamma prime strengthening in cobalt superalloys contribute to their enhanced performance in extreme thermal environments.
Cost and Availability of Cobalt Versus Chromium
Cobalt is significantly more expensive than chromium due to its limited global reserves and complex extraction process, with prices often exceeding $50 per kilogram compared to chromium's $10 per kilogram. Chromium is abundantly available, primarily sourced from large deposits in South Africa and Kazakhstan, making it more cost-effective for superalloy production. The availability of chromium ensures stable supply chains, whereas cobalt faces geopolitical risks, particularly linked to the Democratic Republic of Congo, impacting its price volatility and long-term accessibility.
Environmental and Health Considerations
Cobalt-based superalloys pose significant environmental and health concerns due to the toxic cobalt dust and fumes generated during processing, which can cause respiratory issues and skin sensitization. Chromium in superalloys, while essential for corrosion resistance, may present risks primarily related to hexavalent chromium compounds, known carcinogens that require stringent handling and disposal protocols. Implementing proper protective measures and environmentally responsible waste management is crucial to mitigate the adverse effects associated with the manufacturing and use of cobalt and chromium-containing superalloys.
Future Trends in Cobalt- and Chromium-Based Superalloys
Future trends in cobalt- and chromium-based superalloys emphasize advancements in high-temperature oxidation resistance and mechanical strength for aerospace and power generation applications. Cobalt-based superalloys are being engineered with tailored microstructures to enhance creep resistance, while chromium-based alloys focus on improving corrosion resistance through innovative alloying techniques. Emerging research targets additive manufacturing compatibility and the integration of rare earth elements to optimize performance in extreme environments.
Infographic: Cobalt vs Chromium for Superalloy
 azmater.com
azmater.com