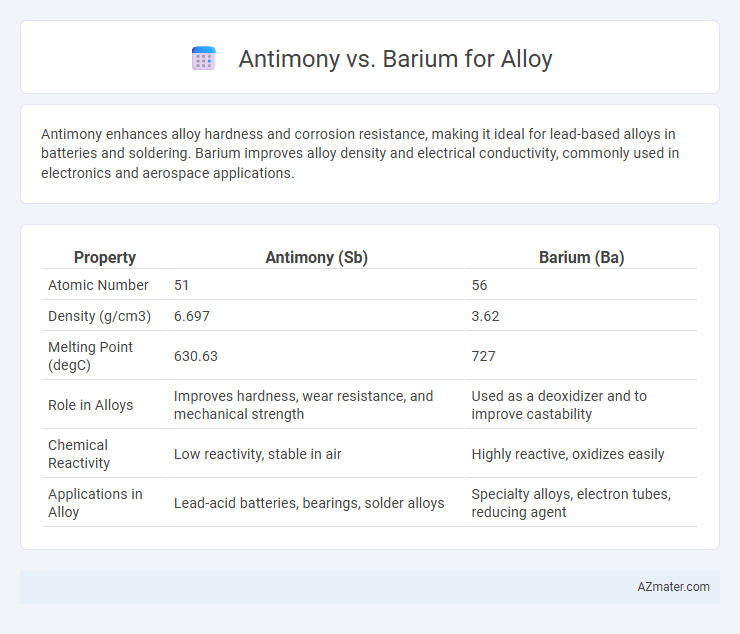
Antimony enhances alloy hardness and corrosion resistance, making it ideal for lead-based alloys in batteries and soldering. Barium improves alloy density and electrical conductivity, commonly used in electronics and aerospace applications.
Table of Comparison
| Property | Antimony (Sb) | Barium (Ba) |
|---|---|---|
| Atomic Number | 51 | 56 |
| Density (g/cm3) | 6.697 | 3.62 |
| Melting Point (degC) | 630.63 | 727 |
| Role in Alloys | Improves hardness, wear resistance, and mechanical strength | Used as a deoxidizer and to improve castability |
| Chemical Reactivity | Low reactivity, stable in air | Highly reactive, oxidizes easily |
| Applications in Alloy | Lead-acid batteries, bearings, solder alloys | Specialty alloys, electron tubes, reducing agent |
Introduction to Antimony and Barium in Alloys
Antimony enhances alloy properties by increasing hardness, strength, and corrosion resistance, commonly used in lead alloys for batteries and bullets. Barium improves alloys by contributing to grain refinement, enhancing wear resistance, and stabilizing microstructures, often found in steel and aluminum alloys. Both elements play crucial roles in tailoring mechanical and chemical characteristics for specific industrial applications.
Chemical and Physical Properties Comparison
Antimony exhibits a high melting point of 630.6degC, a density of 6.697 g/cm3, and is brittle with excellent corrosion resistance, making it ideal for strengthening alloys and improving hardness. Barium, with a melting point of 727degC and a lower density of 3.62 g/cm3, is highly reactive and soft, unsuitable for structural alloy use but valuable in applications requiring chemical reactivity. The chemical stability and hardness of antimony make it preferable for alloying metals like lead and tin, while barium's reactivity limits its use in conventional alloy systems.
Common Industrial Applications
Antimony and barium serve distinct roles in alloy manufacturing, with antimony predominantly used to enhance hardness and mechanical strength in lead-based alloys for batteries, cable sheathing, and bearings. Barium alloys are less common but play a specialized role in improving corrosion resistance and high-temperature stability, particularly in aerospace and automotive components. The choice between antimony and barium depends on specific industrial requirements such as wear resistance, electrical conductivity, and thermal performance.
Alloy Strength and Durability: Antimony vs Barium
Antimony enhances alloy strength by improving hardness and wear resistance, making it ideal for lead-based alloys and soldering applications. Barium contributes to alloy durability through corrosion resistance and high-temperature stability, especially in specialty steel and aluminum alloys. Comparing both, antimony primarily increases mechanical strength, while barium bolsters long-term durability under harsh conditions.
Effects on Corrosion Resistance
Antimony enhances the corrosion resistance of alloys by improving structural integrity and forming a protective oxide layer that inhibits oxidation. Barium, while less common in alloys, can contribute to corrosion resistance by modifying microstructure and reducing impurity segregation, though excessive amounts may lead to brittleness and localized corrosion. The choice between antimony and barium for corrosion resistance depends on the specific alloy system and intended environmental exposure.
Influence on Alloy Melting Points
Antimony increases the melting point of alloys by forming stable intermetallic compounds, which enhances thermal stability and hardness in applications like lead-acid batteries and bearings. Barium, though less common in alloys, tends to lower the melting point slightly due to its metallic properties and larger atomic radius, affecting alloy fluidity and casting ease. Choosing antimony or barium significantly impacts alloy processing temperatures and mechanical properties, making antimony preferable for high-temperature resistance alloys.
Impact on Alloy Machinability
Antimony enhances alloy machinability by improving hardness and wear resistance, resulting in smoother cutting processes and longer tool life. In contrast, barium's impact on machinability is limited but can aid in refining grain structure, which may slightly improve machining consistency. Alloys with antimony typically exhibit better performance in applications requiring precision machining due to its positive effect on chip formation and surface finish.
Cost and Availability Considerations
Antimony is generally more expensive than barium due to its limited global reserves and complex extraction processes, impacting the overall cost of alloys. Barium, being more abundant and easier to source, offers a cost-effective alternative with wider availability. Selecting between antimony and barium for alloy production depends heavily on budget constraints and supply chain stability.
Environmental and Safety Concerns
Antimony alloys pose environmental risks due to their toxicity and potential to bioaccumulate, requiring careful handling and disposal to minimize soil and water contamination. Barium alloys, while less toxic than antimony, still pose health hazards, particularly respiratory issues from dust inhalation and potential contamination in aquatic systems. Both metals demand stringent workplace safety measures and environmental regulations to mitigate adverse impacts during alloy manufacturing and usage.
Choosing Between Antimony and Barium for Specific Alloys
Antimony enhances alloy hardness and wear resistance, making it ideal for lead-acid battery grids and type metal applications, while barium improves high-temperature strength and corrosion resistance, beneficial for specialty steel alloys and automotive parts. Selecting between antimony and barium depends on the desired mechanical properties and operating environment, with antimony preferred for rigidity and barium for thermal stability. Alloy composition analysis and performance requirements guide metallurgists in optimizing element choice for targeted applications.
Infographic: Antimony vs Barium for Alloy
 azmater.com
azmater.com