Antimony enhances alloy hardness, wear resistance, and thermal stability, making it ideal for lead alloys and flame retardants. Aluminum alloys offer superior strength-to-weight ratio, corrosion resistance, and electrical conductivity, widely used in aerospace and automotive industries.
Table of Comparison
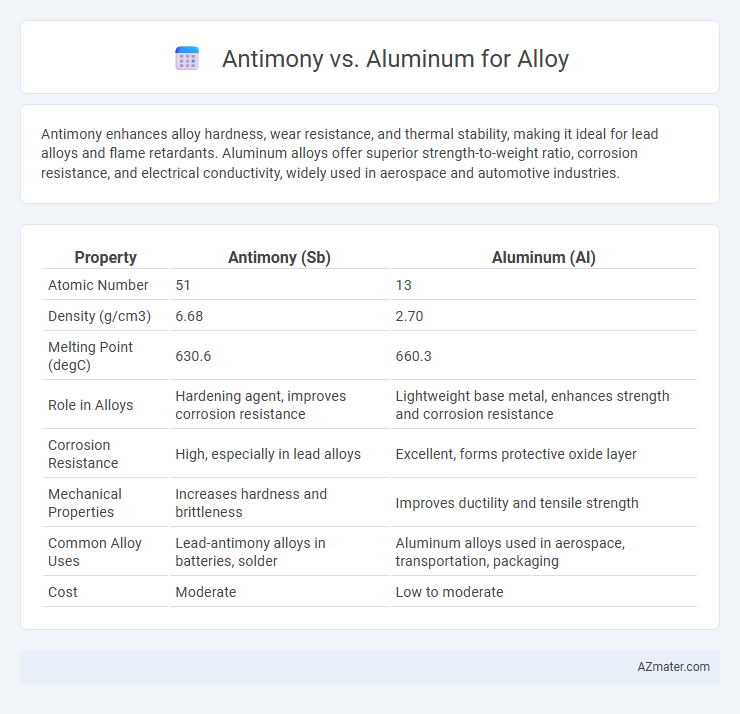
| Property | Antimony (Sb) | Aluminum (Al) |
|---|---|---|
| Atomic Number | 51 | 13 |
| Density (g/cm3) | 6.68 | 2.70 |
| Melting Point (degC) | 630.6 | 660.3 |
| Role in Alloys | Hardening agent, improves corrosion resistance | Lightweight base metal, enhances strength and corrosion resistance |
| Corrosion Resistance | High, especially in lead alloys | Excellent, forms protective oxide layer |
| Mechanical Properties | Increases hardness and brittleness | Improves ductility and tensile strength |
| Common Alloy Uses | Lead-antimony alloys in batteries, solder | Aluminum alloys used in aerospace, transportation, packaging |
| Cost | Moderate | Low to moderate |
Overview of Antimony and Aluminum in Alloying
Antimony is primarily used as a hardening agent in alloys, significantly enhancing the strength and wear resistance of lead-based and tin-based alloys, especially in battery grids and type metals. Aluminum, known for its lightweight and corrosion resistance, is widely alloyed with elements like copper, magnesium, and silicon to improve mechanical properties and thermal stability in aerospace and automotive applications. The combination of aluminum's excellent strength-to-weight ratio with antimony's hardness benefits specialized alloys requiring durability and reduced weight.
Chemical Properties Comparison
Antimony exhibits a higher atomic number (51) and a metalloid nature, contributing to its distinct chemical reactivity and resistance to oxidation compared to aluminum, a lightweight metal with atomic number 13 known for forming a stable oxide layer. In alloy compositions, antimony improves hardness and wear resistance due to its ability to form intermetallic compounds, whereas aluminum enhances corrosion resistance and ductility through the formation of a protective aluminum oxide film. The differing electronegativities and melting points--antimony at 630.6degC and aluminum at 660.3degC--affect the thermal stability and mechanical properties of alloys containing these elements.
Mechanical Strength and Durability
Antimony enhances the mechanical strength and durability of alloys by increasing hardness and resistance to wear, making it ideal for applications requiring robust performance under stress. Aluminum alloys offer a balance of lightweight and moderate strength, with excellent corrosion resistance but generally lower hardness compared to antimony-containing alloys. The inclusion of antimony is particularly beneficial in improving tensile strength and impact resistance, while aluminum alloys excel in environments where weight reduction and oxidation resistance are critical.
Corrosion Resistance Differences
Antimony enhances the corrosion resistance of alloys by improving their ability to withstand oxidation and acidic environments, making it particularly useful in battery grids and bearing alloys. Aluminum offers inherent corrosion resistance due to its natural oxide layer, which protects it from moisture and many chemical reactions, especially in marine or atmospheric conditions. While aluminum provides broad environmental protection, antimony's role is more specialized, improving specific corrosion resistance aspects in lead-based alloys.
Melting Points and Workability
Antimony has a melting point of 630.63degC, significantly lower than aluminum's melting point of 660.3degC, affecting alloy processing temperatures. Aluminum alloys are prized for superior workability due to aluminum's ductility and malleability, while antimony alloys tend to be harder and more brittle, limiting deformation. Consequently, aluminum-based alloys are preferred in applications requiring extensive shaping and forming, whereas antimony alloys are favored for wear-resistant components.
Applications in Industry
Antimony is primarily used in alloys to enhance hardness and mechanical strength, particularly in lead-acid batteries, ammunition, and cable sheathing due to its excellent corrosion resistance and flame retardant properties. Aluminum alloys dominate in aerospace, automotive, and packaging industries because of their lightweight nature, high strength-to-weight ratio, and superior corrosion resistance. Industrial applications leverage antimony for durability in specialized casting and metal consolidation, while aluminum's widespread use is driven by its recyclability and versatility across structural and thermal management contexts.
Environmental and Health Considerations
Antimony alloys pose significant environmental risks due to the toxic nature of antimony, which can contaminate water sources and soil during mining and recycling processes, leading to bioaccumulation and adverse health effects such as respiratory issues and skin irritation. Aluminum alloys are generally considered more environmentally friendly, as aluminum is abundant, recyclable, and has lower toxicity, reducing long-term ecological impact and human health hazards. The energy-intensive extraction of aluminum contributes to environmental concerns, but its recyclability and reduced toxicity offer advantages over antimony in alloy applications.
Cost and Availability Analysis
Antimony, a scarce metalloid primarily sourced from China, is significantly more expensive than aluminum, which is abundant and widely available globally. Aluminum's lower cost and high availability make it ideal for large-scale alloy production, while antimony's higher price limits its use to specialized applications requiring enhanced flame-retardant or hardness properties. Supply chain volatility affects antimony more severely than aluminum, influencing long-term cost stability and accessibility in alloy manufacturing.
Recyclability and Sustainability
Antimony enhances aluminum alloys by improving hardness and corrosion resistance while maintaining high recyclability due to its ability to be efficiently separated and reused without significant degradation. Aluminum alloys containing antimony offer sustainable benefits as aluminum is highly recyclable, requiring only 5% of the energy used for primary production, and the incorporation of antimony does not significantly hinder recycling processes. This combination advances circular economy goals by supporting material longevity and reducing environmental impact through effective resource recovery.
Choosing the Right Alloying Element
Selecting between antimony and aluminum as alloying elements depends on the desired properties of the final alloy; antimony enhances hardness and wear resistance, making it suitable for lead alloys and type metal applications, while aluminum improves corrosion resistance and strength-to-weight ratio, ideal for aerospace and automotive industries. Antimony's high melting point and ability to increase alloy density contrast with aluminum's lightweight nature and excellent thermal conductivity. Consider the application's mechanical requirements, environmental exposure, and manufacturing processes to achieve optimal performance and cost-efficiency.
Infographic: Antimony vs Aluminum for Alloy
 azmater.com
azmater.com