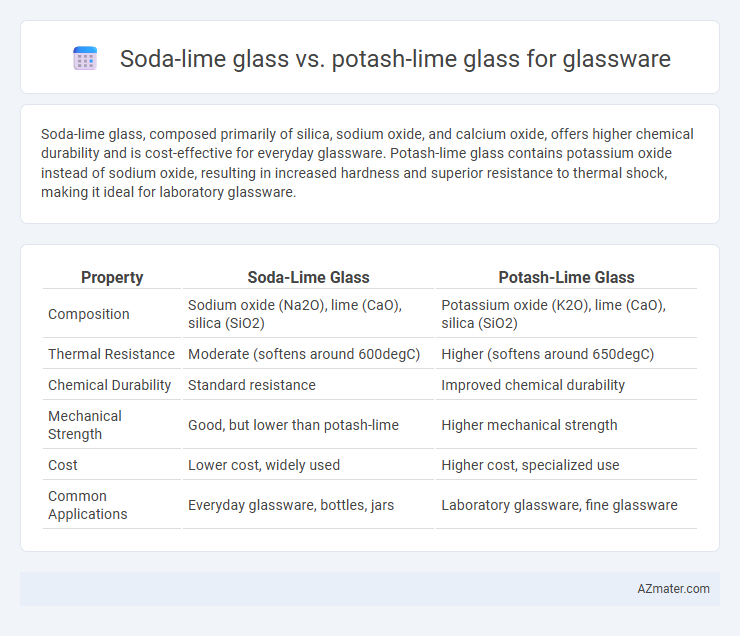
Soda-lime glass, composed primarily of silica, sodium oxide, and calcium oxide, offers higher chemical durability and is cost-effective for everyday glassware. Potash-lime glass contains potassium oxide instead of sodium oxide, resulting in increased hardness and superior resistance to thermal shock, making it ideal for laboratory glassware.
Table of Comparison
| Property | Soda-Lime Glass | Potash-Lime Glass |
|---|---|---|
| Composition | Sodium oxide (Na2O), lime (CaO), silica (SiO2) | Potassium oxide (K2O), lime (CaO), silica (SiO2) |
| Thermal Resistance | Moderate (softens around 600degC) | Higher (softens around 650degC) |
| Chemical Durability | Standard resistance | Improved chemical durability |
| Mechanical Strength | Good, but lower than potash-lime | Higher mechanical strength |
| Cost | Lower cost, widely used | Higher cost, specialized use |
| Common Applications | Everyday glassware, bottles, jars | Laboratory glassware, fine glassware |
Introduction to Soda-lime and Potash-lime Glass
Soda-lime glass, composed primarily of silica, soda, and lime, is the most common type of glass used in glassware due to its affordability, durability, and ease of manufacture. Potash-lime glass, which replaces soda with potash (potassium oxide), offers increased chemical resistance and higher refractive index, making it suitable for decorative and high-quality glassware. Both types exhibit distinct physical and chemical properties that influence their applications in everyday and specialized glass products.
Chemical Composition Differences
Soda-lime glass primarily consists of silica (SiO2) combined with sodium oxide (Na2O) and calcium oxide (CaO), providing durability and ease of manufacture, making it the most common type for glassware. Potash-lime glass substitutes sodium oxide with potassium oxide (K2O), altering thermal and chemical properties, resulting in higher thermal resistance and better chemical durability. The increased potassium content in potash-lime glass enhances scratch resistance and provides a higher refractive index compared to soda-lime glass, influencing the choice of glassware depending on specific application requirements.
Physical Properties Comparison
Soda-lime glass, composed primarily of silica, soda, and lime, offers moderate hardness and good chemical durability, making it resistant to thermal shock but more prone to scratching compared to potash-lime glass. Potash-lime glass contains potassium oxide instead of sodium oxide, resulting in higher refractive index and increased density, which enhances its strength and scratch resistance. The difference in thermal expansion coefficients also means potash-lime glass exhibits better resistance to sudden temperature changes, ideal for more robust glassware applications.
Manufacturing Processes
Soda-lime glass is manufactured by melting silica sand, sodium carbonate (soda ash), and calcium oxide (lime) at high temperatures, resulting in a versatile and cost-effective material widely used in glassware production. Potash-lime glass replaces sodium carbonate with potassium carbonate, requiring higher melting temperatures and different flux ratios that influence the viscosity and workability during manufacturing. The choice between soda-lime and potash-lime glass affects furnace conditions, energy consumption, and final product qualities such as chemical durability and thermal resistance in glassware applications.
Durability and Strength
Soda-lime glass, composed primarily of silica, sodium oxide, and calcium oxide, offers moderate durability and is widely used in everyday glassware due to its cost-effectiveness. Potash-lime glass, containing potassium oxide instead of sodium oxide, provides higher chemical resistance and greater mechanical strength, making it more durable under thermal and physical stress. The increased strength of potash-lime glass reduces the risk of breakage and improves longevity in premium glassware applications.
Thermal and Chemical Resistance
Soda-lime glass offers moderate thermal resistance with a typical strain point around 520degC but is more susceptible to chemical attack by alkalis and acids compared to potash-lime glass. Potash-lime glass exhibits improved chemical resistance due to higher potassium oxide content, enhancing durability against alkali corrosion and making it preferable for glassware exposed to harsh chemicals. Thermal expansion coefficients of potash-lime glass are generally lower, allowing better thermal shock resistance suitable for applications involving rapid temperature changes.
Applications in Glassware
Soda-lime glass is extensively used in everyday glassware such as bottles, jars, and drinking glasses due to its affordability, durability, and good chemical resistance. Potash-lime glass, valued for its higher refractive index and enhanced clarity, is predominantly employed in decorative glassware, fine glass art, and luxury table settings. Both types offer unique benefits, with soda-lime glass excelling in mass production and potash-lime glass preferred for aesthetic and optical quality in premium glassware applications.
Cost and Availability
Soda-lime glass is the most commonly used glass in glassware production due to its low cost and widespread availability, making it an economical choice for manufacturers. Potash-lime glass, while offering better chemical durability, is less common and generally more expensive because potash is a costlier raw material and less abundantly sourced. The price difference and material availability significantly influence the selection between soda-lime and potash-lime glass in large-scale glassware manufacturing.
Environmental Impact
Soda-lime glass, the most common type used in glassware, features a lower melting point, reducing energy consumption during production and contributing to a smaller carbon footprint compared to potash-lime glass. Potash-lime glass, with higher potassium oxide content, often requires more energy to melt and produce, leading to increased greenhouse gas emissions. Recycling rates for soda-lime glass surpass those of potash-lime glass, enhancing its environmental sustainability through material reuse and waste reduction.
Choosing the Right Glass for Your Needs
Soda-lime glass, composed mainly of silica, soda, and lime, is the most common type used in glassware due to its affordability, durability, and excellent clarity, making it ideal for everyday use such as drinkware and storage containers. Potash-lime glass, enriched with potassium oxide instead of soda, offers superior chemical resistance and thermal stability, making it preferable for laboratory glassware or high-end glassware requiring better heat tolerance and less thermal expansion. Selecting between soda-lime and potash-lime glass depends on the intended application, budget, and required durability, with potash-lime glass providing enhanced performance for specialized uses, while soda-lime glass remains the best all-purpose choice for general household glassware.
Infographic: Soda-lime glass vs Potash-lime glass for Glassware
 azmater.com
azmater.com