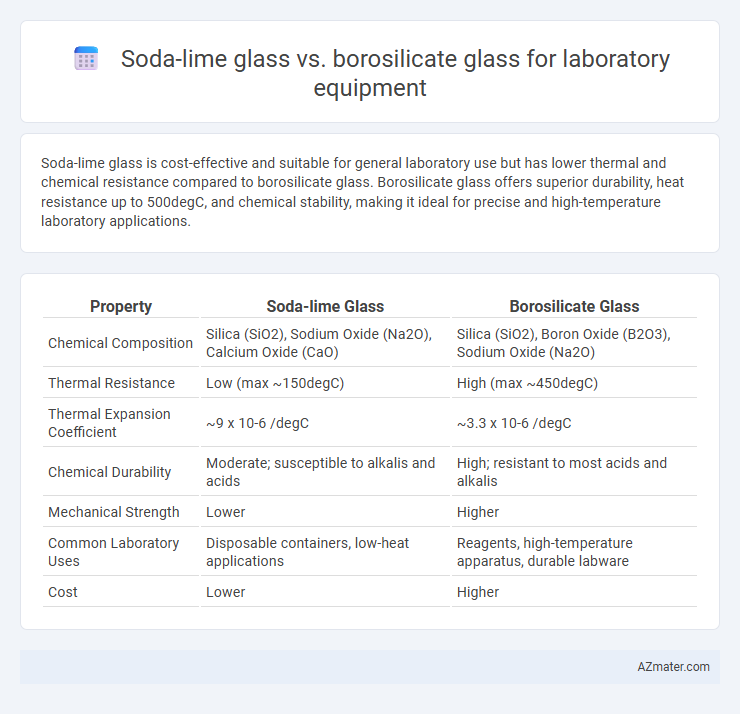
Soda-lime glass is cost-effective and suitable for general laboratory use but has lower thermal and chemical resistance compared to borosilicate glass. Borosilicate glass offers superior durability, heat resistance up to 500degC, and chemical stability, making it ideal for precise and high-temperature laboratory applications.
Table of Comparison
| Property | Soda-lime Glass | Borosilicate Glass |
|---|---|---|
| Chemical Composition | Silica (SiO2), Sodium Oxide (Na2O), Calcium Oxide (CaO) | Silica (SiO2), Boron Oxide (B2O3), Sodium Oxide (Na2O) |
| Thermal Resistance | Low (max ~150degC) | High (max ~450degC) |
| Thermal Expansion Coefficient | ~9 x 10-6 /degC | ~3.3 x 10-6 /degC |
| Chemical Durability | Moderate; susceptible to alkalis and acids | High; resistant to most acids and alkalis |
| Mechanical Strength | Lower | Higher |
| Common Laboratory Uses | Disposable containers, low-heat applications | Reagents, high-temperature apparatus, durable labware |
| Cost | Lower | Higher |
Introduction to Laboratory Glassware Materials
Laboratory glassware primarily uses soda-lime and borosilicate glass due to their distinct physical and chemical properties. Soda-lime glass, composed mainly of silica, sodium oxide, and calcium oxide, offers affordability and ease of manufacture but has lower thermal resistance and chemical durability. Borosilicate glass, containing silica and boron trioxide, exhibits superior thermal shock resistance, chemical stability, and is preferred for precise laboratory applications requiring high durability and resistance to temperature changes.
Chemical Composition of Soda-lime and Borosilicate Glass
Soda-lime glass primarily consists of approximately 70-75% silica (SiO2), 12-15% sodium oxide (Na2O), and 10-15% calcium oxide (CaO), providing good thermal and chemical stability at low cost. Borosilicate glass contains about 70-80% silica, 7-13% boron oxide (B2O3), and lower amounts of alkali oxides, granting superior resistance to thermal shock and chemical corrosion. The inclusion of boron oxide in borosilicate glass significantly reduces its coefficient of thermal expansion compared to soda-lime glass, making it ideal for precise laboratory applications.
Thermal Properties Comparison
Soda-lime glass exhibits a higher coefficient of thermal expansion, approximately 9 x 10-6 /degC, making it more susceptible to thermal shock and less ideal for rapid temperature changes in laboratory settings. Borosilicate glass has a significantly lower thermal expansion coefficient, around 3.3 x 10-6 /degC, providing superior resistance to thermal shock and better stability under high-temperature fluctuations. This enhanced thermal resilience makes borosilicate glass the preferred choice for precision laboratory equipment such as beakers, flasks, and condensers.
Resistance to Chemical Corrosion
Borosilicate glass exhibits superior resistance to chemical corrosion compared to soda-lime glass, making it the preferred choice for laboratory equipment exposed to aggressive chemicals and thermal stress. Soda-lime glass is more susceptible to alkaline and acidic attack, leading to potential contamination and reduced durability in chemical environments. Laboratories prioritize borosilicate glass for its low coefficient of thermal expansion and high chemical inertness, ensuring reliability and longevity in experimental applications.
Mechanical Strength and Durability
Borosilicate glass exhibits superior mechanical strength and durability compared to soda-lime glass, making it highly resistant to thermal shock and mechanical stress in laboratory settings. Its low coefficient of thermal expansion allows borosilicate glass to withstand rapid temperature changes without cracking, unlike soda-lime glass, which is more prone to breakage under thermal and mechanical strain. These properties make borosilicate glass the preferred choice for laboratory equipment requiring enhanced durability and long-term performance.
Cost and Availability
Soda-lime glass is significantly more cost-effective and widely available compared to borosilicate glass, making it the preferred choice for general laboratory glassware and large-scale applications. Borosilicate glass, despite its higher price, offers superior thermal resistance and chemical durability, justifying its use in specialized laboratory equipment where these properties are critical. The widespread production and lower manufacturing costs of soda-lime glass ensure easier procurement and replacement in most laboratory settings.
Applications in Laboratory Settings
Soda-lime glass is commonly used in laboratory equipment for general-purpose containers, beakers, and test tubes due to its affordability and adequate chemical resistance to non-corrosive substances. Borosilicate glass is preferred in laboratories requiring thermal resistance and durability, such as in the manufacturing of flasks, pipettes, and condensers, because it withstands rapid temperature changes and corrosive chemicals. The choice between soda-lime and borosilicate glass depends on the specific laboratory application, with borosilicate being essential for high-precision and high-temperature experiments.
Safety Considerations
Soda-lime glass, commonly used in laboratory equipment, offers good chemical resistance but is more prone to thermal shock and breakage compared to borosilicate glass. Borosilicate glass excels in safety due to its low thermal expansion coefficient, allowing it to withstand rapid temperature changes without cracking, making it ideal for high-temperature experiments. For rigorous laboratory applications, borosilicate glass enhances safety by reducing the risk of hazardous glass failure during heating and cooling cycles.
Environmental Impact of Production and Disposal
Soda-lime glass production involves lower energy consumption and emits fewer greenhouse gases compared to borosilicate glass, which requires higher temperatures and raw material processing. In disposal, soda-lime glass is more readily recyclable in conventional glass recycling streams, whereas borosilicate glass's chemical durability complicates recycling, often necessitating specialized handling. Environmental impact assessments favor soda-lime glass for lower carbon footprint and easier end-of-life management in laboratory equipment applications.
Choosing the Right Glass for Your Laboratory Needs
Soda-lime glass offers cost-effectiveness and adequate chemical resistance for routine laboratory applications, making it suitable for short-term use and general-purpose containers. Borosilicate glass provides superior thermal resistance and durability, ideal for experiments involving rapid temperature changes or corrosive chemicals. Choosing the right glass depends on the specific laboratory requirements, balancing budget constraints with the need for thermal and chemical stability.
Infographic: Soda-lime glass vs Borosilicate glass for Laboratory equipment
 azmater.com
azmater.com