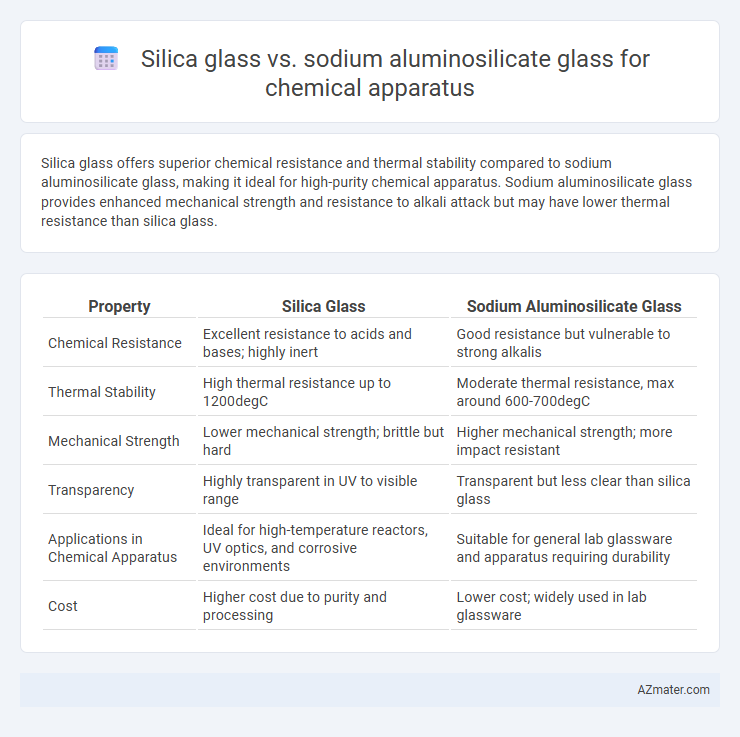
Silica glass offers superior chemical resistance and thermal stability compared to sodium aluminosilicate glass, making it ideal for high-purity chemical apparatus. Sodium aluminosilicate glass provides enhanced mechanical strength and resistance to alkali attack but may have lower thermal resistance than silica glass.
Table of Comparison
| Property | Silica Glass | Sodium Aluminosilicate Glass |
|---|---|---|
| Chemical Resistance | Excellent resistance to acids and bases; highly inert | Good resistance but vulnerable to strong alkalis |
| Thermal Stability | High thermal resistance up to 1200degC | Moderate thermal resistance, max around 600-700degC |
| Mechanical Strength | Lower mechanical strength; brittle but hard | Higher mechanical strength; more impact resistant |
| Transparency | Highly transparent in UV to visible range | Transparent but less clear than silica glass |
| Applications in Chemical Apparatus | Ideal for high-temperature reactors, UV optics, and corrosive environments | Suitable for general lab glassware and apparatus requiring durability |
| Cost | Higher cost due to purity and processing | Lower cost; widely used in lab glassware |
Introduction to Glass Types in Chemical Apparatus
Silica glass offers exceptional thermal stability and chemical resistance, making it ideal for high-temperature chemical apparatus where purity and durability are critical. Sodium aluminosilicate glass, while less resistant to extreme heat, provides improved mechanical strength and resistance to alkali environments, suitable for applications involving caustic substances. Both glass types serve distinct roles in chemical apparatus, with silica glass preferred for precision and thermal resilience, and sodium aluminosilicate glass favored for enhanced toughness and chemical versatility.
Chemical Composition: Silica Glass vs Sodium Aluminosilicate Glass
Silica glass primarily consists of silicon dioxide (SiO2) with high purity, providing excellent chemical resistance and thermal stability crucial for chemical apparatus. Sodium aluminosilicate glass incorporates significant amounts of sodium oxide (Na2O) and aluminum oxide (Al2O3) alongside silicon dioxide, enhancing mechanical strength but reducing chemical durability compared to pure silica glass. The differing chemical compositions influence their suitability for various chemical environments, with silica glass being preferred for highly corrosive or high-temperature conditions.
Thermal Stability Comparison
Silica glass exhibits exceptional thermal stability with a high melting point around 1713degC, making it ideal for high-temperature chemical apparatus applications. Sodium aluminosilicate glass, with a lower melting point near 1100degC, offers moderate thermal resistance but enhanced mechanical strength and chemical durability. The superior thermal stability of silica glass ensures minimal deformation and better resistance to thermal shock under extreme laboratory conditions.
Chemical Resistance and Corrosion Behaviors
Silica glass exhibits superior chemical resistance and corrosion resistance compared to sodium aluminosilicate glass, making it highly suitable for chemical apparatus exposed to aggressive acids and alkalis. Sodium aluminosilicate glass offers moderate durability but is more prone to alkali attack and degradation in highly corrosive environments. The high purity and network structure of silica glass significantly reduce ion exchange and leaching, enhancing its longevity in harsh chemical processes.
Mechanical Strength and Durability
Silica glass exhibits superior mechanical strength and exceptional thermal and chemical durability, making it ideal for demanding chemical apparatus that require resistance to high temperatures and aggressive chemicals. Sodium aluminosilicate glass offers enhanced mechanical toughness and improved resistance to thermal shock compared to standard sodalime glass, but it generally has lower chemical durability than pure silica glass due to its alumina and sodium content. The choice between these glasses for chemical apparatus depends on the balance of required mechanical strength, thermal resistance, and chemical durability in specific laboratory or industrial applications.
Optical Clarity and Transparency Differences
Silica glass exhibits superior optical clarity with minimal light absorption and high transparency across ultraviolet to infrared wavelengths, making it ideal for precise optical applications in chemical apparatus. Sodium aluminosilicate glass, while chemically durable and resistant to thermal shock, offers lower optical clarity due to greater light scattering and absorption caused by its complex aluminosilicate composition. The differences in their microstructure directly impact transparency, with silica glass maintaining higher transmittance essential for accurate visual monitoring in chemical processes.
Cost and Availability for Laboratory Use
Silica glass offers superior chemical resistance and thermal stability but is generally more expensive and less widely available compared to sodium aluminosilicate glass for laboratory apparatus. Sodium aluminosilicate glass provides a cost-effective alternative with good durability and better availability, making it suitable for routine lab uses where extreme chemical resistance is not critical. Laboratories prioritizing budget and supply ease often select sodium aluminosilicate glass, while those requiring high-performance apparatus opt for the more costly silica glass despite limited availability.
Practical Applications in Laboratory Apparatus
Silica glass excels in laboratory apparatus requiring high thermal resistance and chemical inertness, making it ideal for reaction vessels, crucibles, and high-temperature applications where purity and durability are critical. Sodium aluminosilicate glass offers enhanced mechanical strength and chemical durability in moderately corrosive environments, commonly used for standard labware such as beakers and bottles due to its cost-effectiveness and resistance to alkali attack. Practical applications favor silica glass for high-precision and high-temperature operations, while sodium aluminosilicate glass suits general-purpose chemical containers with less stringent thermal demands.
Maintenance and Lifespan Considerations
Silica glass offers superior chemical resistance and thermal stability, making it highly durable and requiring minimal maintenance for chemical apparatus, contributing to a longer lifespan compared to sodium aluminosilicate glass. Sodium aluminosilicate glass, while more economical, is prone to alkali leaching and surface degradation under harsh chemical environments, necessitating more frequent inspections and maintenance. The enhanced durability and inertness of silica glass significantly reduce downtime and replacement costs in laboratory and industrial settings.
Choosing the Right Glass for Chemical Apparatus
Silica glass offers exceptional thermal resistance and chemical inertness, making it ideal for high-temperature chemical apparatus requiring durability and purity. Sodium aluminosilicate glass provides improved mechanical strength and chemical resistance at moderate temperatures, suitable for apparatus exposed to alkalis or mechanical stress. Selecting the right glass depends on the operational temperature, chemical compatibility, and mechanical demands of the intended chemical processes.
Infographic: Silica glass vs Sodium aluminosilicate glass for Chemical apparatus
 azmater.com
azmater.com