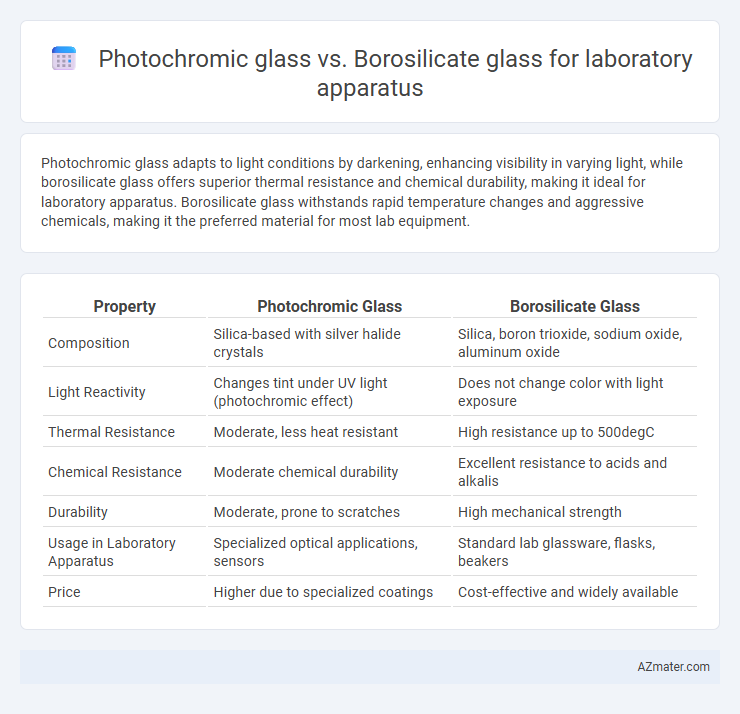
Photochromic glass adapts to light conditions by darkening, enhancing visibility in varying light, while borosilicate glass offers superior thermal resistance and chemical durability, making it ideal for laboratory apparatus. Borosilicate glass withstands rapid temperature changes and aggressive chemicals, making it the preferred material for most lab equipment.
Table of Comparison
| Property | Photochromic Glass | Borosilicate Glass |
|---|---|---|
| Composition | Silica-based with silver halide crystals | Silica, boron trioxide, sodium oxide, aluminum oxide |
| Light Reactivity | Changes tint under UV light (photochromic effect) | Does not change color with light exposure |
| Thermal Resistance | Moderate, less heat resistant | High resistance up to 500degC |
| Chemical Resistance | Moderate chemical durability | Excellent resistance to acids and alkalis |
| Durability | Moderate, prone to scratches | High mechanical strength |
| Usage in Laboratory Apparatus | Specialized optical applications, sensors | Standard lab glassware, flasks, beakers |
| Price | Higher due to specialized coatings | Cost-effective and widely available |
Introduction to Laboratory Glassware Materials
Photochromic glass in laboratory apparatus offers dynamic light modulation by changing opacity in response to UV exposure, enhancing protection during experiments involving intense light or UV radiation. Borosilicate glass, renowned for its high thermal and chemical resistance, remains the industry standard for durable and reliable laboratory glassware such as beakers, flasks, and test tubes. Selecting between photochromic and borosilicate glass depends on specific laboratory requirements, with borosilicate excelling in general-purpose applications and photochromic glass providing specialized utility in light-sensitive environments.
Overview of Photochromic Glass
Photochromic glass contains molecules that change color when exposed to ultraviolet (UV) light, offering dynamic light modulation beneficial for laboratory environments needing variable light control. Unlike borosilicate glass, which is prized for its thermal resistance and chemical durability, photochromic glass excels in applications requiring adaptive optical properties. Its ability to reduce UV exposure while maintaining clarity makes it suitable for sensitive experiments where light-dependent reactions must be precisely managed.
Overview of Borosilicate Glass
Borosilicate glass, known for its exceptional thermal resistance and chemical durability, is widely used in laboratory apparatus to withstand rapid temperature changes and corrosive substances. Its low thermal expansion coefficient minimizes the risk of breakage during heating or cooling processes, making it ideal for beakers, test tubes, and condensers. Unlike photochromic glass, which alters its properties based on light exposure, borosilicate glass maintains consistent transparency and structural integrity under harsh lab conditions.
Chemical Resistance: Photochromic vs Borosilicate Glass
Borosilicate glass exhibits superior chemical resistance compared to photochromic glass, making it the preferred choice for laboratory apparatus exposed to aggressive acids, alkalis, and solvents. Photochromic glass, while beneficial for light-sensitive applications, typically lacks the durability to withstand prolonged contact with harsh chemicals without degradation. The inherent resistance of borosilicate glass to chemical corrosion ensures greater longevity and reliability in laboratory environments requiring frequent chemical handling.
Thermal Properties and Heat Tolerance Comparison
Photochromic glass offers moderate thermal resistance with the ability to darken upon UV exposure, but its heat tolerance is generally lower than borosilicate glass, which is renowned for its high thermal resistance and low thermal expansion coefficient. Borosilicate glass tolerates rapid temperature changes up to approximately 400degC, making it ideal for laboratory apparatus requiring heat durability and resistance to thermal shock. Photochromic glass is better suited for applications prioritizing light sensitivity over extreme heat endurance, whereas borosilicate glass remains the preferred choice for high-temperature laboratory environments.
Optical Qualities and Light Sensitivity
Photochromic glass offers dynamic light sensitivity by darkening upon UV exposure, enabling automatic adjustment to varying light conditions in laboratory settings, enhancing optical clarity and reducing glare. Borosilicate glass features superior optical clarity and resistance to thermal and chemical stresses but lacks light-responsive properties, making it stable yet static under consistent light exposure. The choice hinges on experimental needs: photochromic glass optimizes light adaptability, while borosilicate glass ensures consistent transparency and durability.
Durability and Mechanical Strength
Photochromic glass offers moderate durability but is primarily designed for light-reactive properties rather than mechanical strength, making it less suitable for heavy-duty laboratory apparatus. Borosilicate glass features exceptional durability and high mechanical strength due to its low thermal expansion coefficient and resistance to thermal shock, making it the preferred choice for laboratory glassware subjected to rapid temperature changes and physical stress. The superior chemical resistance and robustness of borosilicate glass ensure reliable performance and longevity in demanding lab environments.
Common Laboratory Applications
Photochromic glass adapts its tint in response to UV light, making it ideal for laboratory apparatus requiring dynamic light control, such as protective eyewear and light-sensitive reaction vessels. Borosilicate glass offers exceptional thermal resistance and chemical durability, commonly used for beakers, flasks, and condensers that undergo high-temperature processes and exposure to corrosive chemicals. Both types serve distinct roles: photochromic glass enhances safety and light regulation, while borosilicate glass ensures structural integrity and chemical compatibility in rigorous lab environments.
Cost-Effectiveness and Availability
Photochromic glass offers advanced UV protection but tends to be more expensive and less readily available than borosilicate glass, which is widely used due to its durability and chemical resistance. Borosilicate glass is highly cost-effective for laboratory apparatus because it is mass-produced and compatible with high-temperature applications. Availability of borosilicate glass ensures a broad selection of standardized laboratory equipment, whereas photochromic glass is typically specialized and limited in supply.
Choosing the Right Glass for Laboratory Use
Photochromic glass offers adaptive light filtering by darkening in response to UV exposure, enhancing visibility and protection during experiments involving intense light. Borosilicate glass is favored for laboratory apparatus due to its exceptional thermal resistance, chemical durability, and low thermal expansion, making it ideal for high-temperature reactions and precise measurements. Selecting between photochromic and borosilicate glass depends on the need for UV protection versus thermal and chemical stability in laboratory applications.
Infographic: Photochromic glass vs Borosilicate glass for Laboratory apparatus
 azmater.com
azmater.com