Opal glass offers excellent chemical resistance and uniform translucency, making it ideal for precise light diffusion in laboratory ware. Borosilicate glass provides superior thermal shock resistance and durability, preferred for high-temperature experiments and chemical stability.
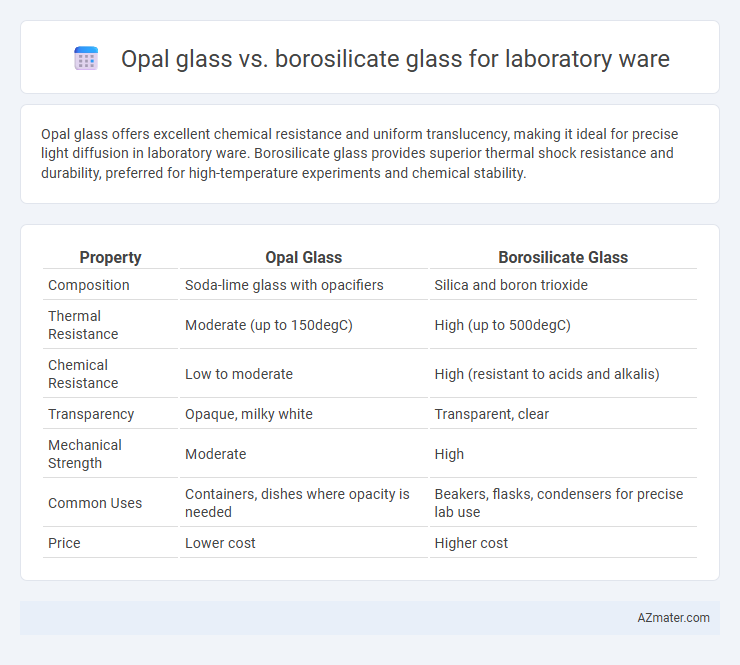
Table of Comparison
| Property | Opal Glass | Borosilicate Glass |
|---|---|---|
| Composition | Soda-lime glass with opacifiers | Silica and boron trioxide |
| Thermal Resistance | Moderate (up to 150degC) | High (up to 500degC) |
| Chemical Resistance | Low to moderate | High (resistant to acids and alkalis) |
| Transparency | Opaque, milky white | Transparent, clear |
| Mechanical Strength | Moderate | High |
| Common Uses | Containers, dishes where opacity is needed | Beakers, flasks, condensers for precise lab use |
| Price | Lower cost | Higher cost |
Introduction to Laboratory Glassware Materials
Opal glass and borosilicate glass are common materials used in laboratory glassware, each with distinct properties suitable for various applications. Borosilicate glass offers high thermal resistance and chemical durability, making it ideal for handling extreme temperature changes and corrosive substances. Opal glass, characterized by its milky white appearance due to added minerals, provides enhanced light diffusion and aesthetic appeal but generally lacks the thermal and chemical resistance of borosilicate.
What is Opal Glass?
Opal glass is a type of laboratory glassware known for its milky white, translucent appearance, created by incorporating opacifying agents into the glass composition. It offers excellent chemical resistance and thermal stability, making it suitable for applications requiring protection from light or heat. Compared to borosilicate glass, opal glass provides enhanced opacity but generally has lower resistance to thermal shock.
What is Borosilicate Glass?
Borosilicate glass, known for its exceptional thermal resistance and chemical durability, is a preferred choice for laboratory ware that requires high precision and safety. Unlike opal glass, which is opaque and primarily used for aesthetic or light-diffusing purposes, borosilicate glass withstands rapid temperature changes and corrosive chemicals, making it ideal for scientific experiments and industrial applications. Its low thermal expansion coefficient ensures durability and minimizes the risk of breakage during heating or cooling processes in laboratory settings.
Key Physical Properties Comparison
Opal glass exhibits lower thermal shock resistance and a higher thermal expansion coefficient compared to borosilicate glass, making it less suitable for rapid temperature changes in laboratory settings. Borosilicate glass offers superior chemical durability and withstands temperatures up to 450degC, while opal glass typically endures lower maximum temperatures and has a more opaque appearance due to its silica-alumina composition. The enhanced mechanical strength and resistance to thermal stress of borosilicate glass make it the preferred choice for high-precision laboratory ware.
Chemical Resistance: Opal vs Borosilicate
Borosilicate glass offers superior chemical resistance compared to opal glass, particularly against strong acids, bases, and organic solvents commonly encountered in laboratory settings. Opal glass, while providing aesthetic opacity and moderate chemical durability, is more susceptible to etching and degradation under harsh chemical exposure. Laboratories often prefer borosilicate glassware for applications demanding rigorous chemical stability and repeatable performance.
Thermal Shock Resistance: Which Performs Better?
Borosilicate glass exhibits superior thermal shock resistance compared to opal glass, making it the preferred choice for laboratory ware subjected to rapid temperature changes. Its low coefficient of thermal expansion (approximately 3.3 x 10^-6 /degC) enables it to withstand sudden heat spikes without cracking. Opal glass, while aesthetically appealing and translucent, has a higher expansion coefficient and is more prone to thermal stress fractures.
Common Laboratory Applications
Opal glass is commonly used in laboratory ware for applications requiring diffused light transmission and chemical resistance, such as in weighing bottles and drying tubes. Borosilicate glass is favored for its superior thermal resistance, mechanical strength, and chemical inertness, making it ideal for reaction vessels, condensers, and volumetric flasks. Both glasses serve critical roles in laboratories: opal glass excels in light-sensitive environments, while borosilicate glass dominates high-temperature and corrosive chemical processes.
Cost and Availability Considerations
Opal glass laboratory ware generally has a lower cost compared to borosilicate glass due to its simpler manufacturing process and widespread availability, making it a budget-friendly choice for basic applications. Borosilicate glass, known for its superior thermal resistance and chemical durability, tends to be more expensive and may have limited availability in some regions, impacting procurement timelines. Laboratories must balance cost constraints with performance needs, as borosilicate glass often justifies its higher price through enhanced longevity and reduced replacement frequency.
Environmental and Safety Factors
Opal glass offers enhanced opacity and chemical resistance, reducing light-sensitive reaction risks and improving safety in laboratory environments. Borosilicate glass is highly durable, thermally resistant, and less prone to thermal shock, promoting long-term sustainability by minimizing breakage waste. Both materials contribute to safer lab conditions, but borosilicate glass's recyclability and longevity make it the more environmentally friendly choice.
Choosing the Right Glassware for Your Lab
Choosing the right glassware for your lab depends on the specific demands of your experiments, with opal glass offering excellent chemical resistance and translucency for applications requiring diffused light, while borosilicate glass provides superior thermal shock resistance and durability for high-temperature procedures. Opal glass is ideal for general-purpose use and visible light filtration, whereas borosilicate glass excels in handling rapid temperature changes and corrosive chemicals, making it the preferred choice for rigorous laboratory environments. Careful consideration of heat resistance, chemical compatibility, and optical properties ensures optimal performance and safety in your lab operations.
Infographic: Opal glass vs Borosilicate glass for Laboratory ware
 azmater.com
azmater.com