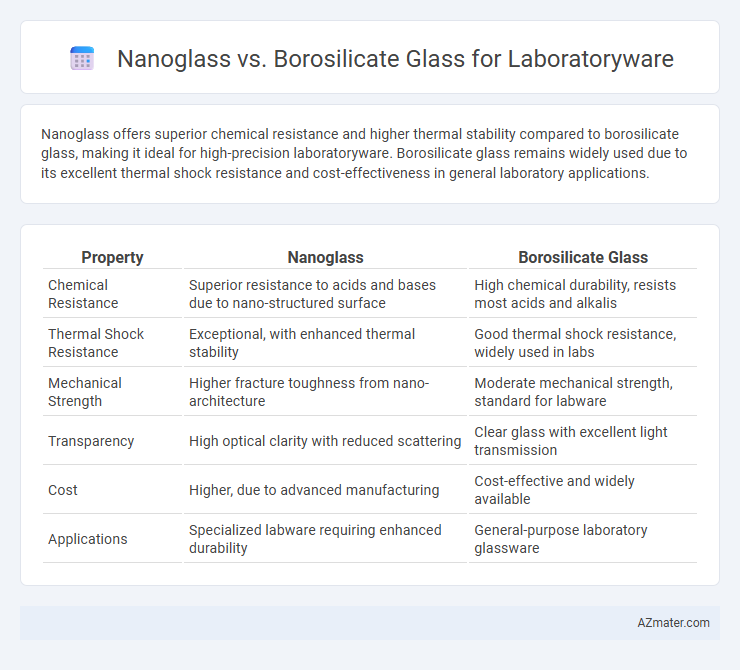
Nanoglass offers superior chemical resistance and higher thermal stability compared to borosilicate glass, making it ideal for high-precision laboratoryware. Borosilicate glass remains widely used due to its excellent thermal shock resistance and cost-effectiveness in general laboratory applications.
Table of Comparison
| Property | Nanoglass | Borosilicate Glass |
|---|---|---|
| Chemical Resistance | Superior resistance to acids and bases due to nano-structured surface | High chemical durability, resists most acids and alkalis |
| Thermal Shock Resistance | Exceptional, with enhanced thermal stability | Good thermal shock resistance, widely used in labs |
| Mechanical Strength | Higher fracture toughness from nano-architecture | Moderate mechanical strength, standard for labware |
| Transparency | High optical clarity with reduced scattering | Clear glass with excellent light transmission |
| Cost | Higher, due to advanced manufacturing | Cost-effective and widely available |
| Applications | Specialized labware requiring enhanced durability | General-purpose laboratory glassware |
Introduction to Laboratory Glassware Materials
Nanoglass and borosilicate glass are pivotal materials in laboratory glassware, each offering distinct advantages. Borosilicate glass is renowned for its exceptional thermal resistance and chemical durability, making it a standard choice for high-temperature applications and corrosive reagents. Nanoglass incorporates nanoscale reinforcements that enhance mechanical strength and reduce breakage, providing innovative solutions for precision and longevity in laboratory environments.
What is Nanoglass?
Nanoglass is a cutting-edge type of glass incorporating nanoscale particles to enhance its mechanical strength, chemical resistance, and thermal stability, making it highly suitable for advanced laboratoryware applications. Unlike traditional borosilicate glass, known for its excellent thermal resistance and chemical durability, nanoglass offers superior fracture toughness and scratch resistance due to its unique nanostructure. This innovative material enables laboratories to handle more aggressive chemicals and rapid temperature changes without compromising safety or accuracy.
Overview of Borosilicate Glass
Borosilicate glass is a highly durable laboratory material composed primarily of silica and boron trioxide, known for its exceptional thermal resistance and low thermal expansion coefficient. Its chemical inertness makes it ideal for handling corrosive substances and withstanding rapid temperature changes without cracking. Widely used in scientific labs, borosilicate glass offers superior resistance to thermal shock compared to standard glass types, ensuring reliable performance in various experimental conditions.
Chemical Resistance: Nanoglass vs Borosilicate
Nanoglass offers superior chemical resistance compared to borosilicate glass, resisting a wider range of aggressive acids and alkalis without degradation. Borosilicate glass, while highly resistant to thermal shock and many chemicals, can be vulnerable to hydrofluoric acid and strong alkaline solutions over time. Laboratories requiring maximum durability in highly corrosive environments often prefer nanoglass for its enhanced inertness and longevity.
Thermal Stability and Performance Comparison
Nanoglass exhibits superior thermal stability compared to borosilicate glass, maintaining structural integrity at temperatures exceeding 1,200degC, while borosilicate glass typically withstands up to 820degC. Its enhanced thermal shock resistance reduces the risk of cracking during rapid temperature changes, making nanoglass ideal for high-temperature laboratory processes such as crucibles and reactors. Performance tests demonstrate that nanoglass offers longer service life and improved chemical durability under extreme thermal cycling, outperforming conventional borosilicate in demanding lab applications.
Mechanical Strength: Durability and Breakage
Nanoglass laboratoryware exhibits superior mechanical strength compared to borosilicate glass, offering enhanced durability and resistance to breakage under thermal and mechanical stress. The innovative nanostructure of nanoglass significantly reduces the likelihood of cracks and fractures, making it ideal for rigorous laboratory environments. Borosilicate glass, while resistant to thermal shock, remains more prone to chipping and breakage during handling or impact.
Cost Efficiency and Availability
Nanoglass offers superior chemical resistance and durability compared to borosilicate glass, but typically comes with a higher price tag and limited availability due to advanced manufacturing processes. Borosilicate glass remains the preferred choice for laboratoryware because of its cost efficiency, widespread availability, and proven thermal shock resistance. Laboratories prioritize borosilicate for routine applications, reserving nanoglass for specialized uses where enhanced material performance justifies the additional cost.
Applications in Lab Settings
Nanoglass offers enhanced chemical resistance and higher thermal stability, making it ideal for high-precision analytical procedures and working with aggressive solvents. Borosilicate glass remains a versatile choice for general laboratory applications due to its excellent thermal shock resistance and durability under routine heating and cooling cycles. Laboratories preferring Nanoglass benefit from reduced contamination risks and longer lifespan in specialized experiments, while Borosilicate glass suits standard reagent storage, mixing, and heating tasks.
Environmental Impact and Sustainability
Nanoglass laboratoryware offers enhanced durability and chemical resistance, significantly reducing breakage and waste compared to traditional borosilicate glass. The manufacturing process of nanoglass utilizes less energy and generates fewer emissions, promoting a lower carbon footprint for laboratory operations. Borosilicate glass, while recyclable, often requires higher energy inputs during production and is more prone to fracture, leading to increased resource consumption and environmental burden over time.
Choosing the Right Glassware for Your Laboratory
Nanoglass offers superior chemical resistance and enhanced thermal stability compared to traditional borosilicate glass, making it ideal for laboratories requiring highly precise reactions and durability under extreme conditions. Borosilicate glass remains a cost-effective option with excellent thermal shock resistance and widespread compatibility for general laboratory applications. Selecting glassware depends on balancing budget constraints with performance needs, prioritizing nanoglass for high-precision analytical tasks and borosilicate for routine experimentation.
Infographic: Nanoglass vs Borosilicate glass for Laboratoryware
 azmater.com
azmater.com