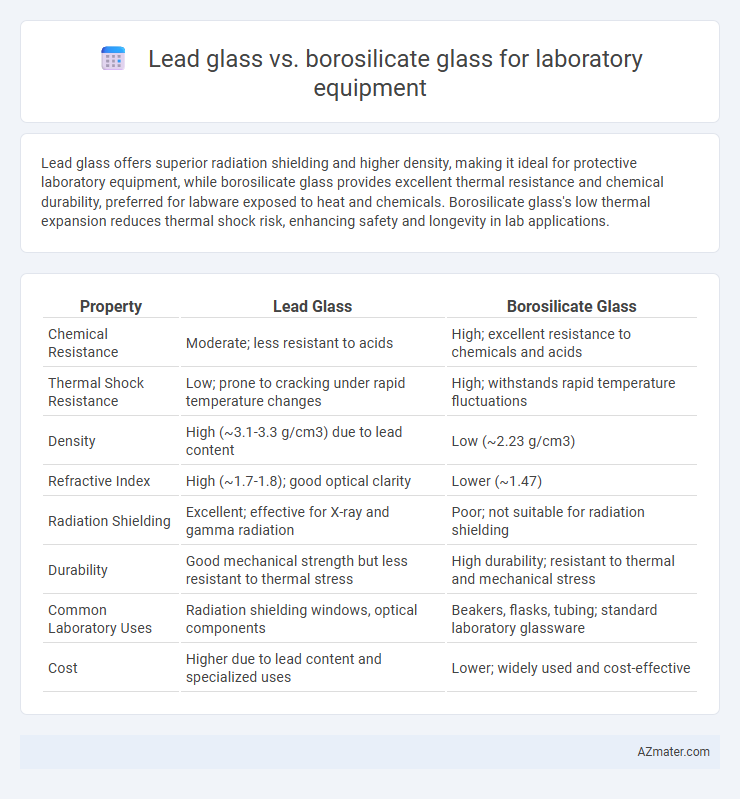
Lead glass offers superior radiation shielding and higher density, making it ideal for protective laboratory equipment, while borosilicate glass provides excellent thermal resistance and chemical durability, preferred for labware exposed to heat and chemicals. Borosilicate glass's low thermal expansion reduces thermal shock risk, enhancing safety and longevity in lab applications.
Table of Comparison
| Property | Lead Glass | Borosilicate Glass |
|---|---|---|
| Chemical Resistance | Moderate; less resistant to acids | High; excellent resistance to chemicals and acids |
| Thermal Shock Resistance | Low; prone to cracking under rapid temperature changes | High; withstands rapid temperature fluctuations |
| Density | High (~3.1-3.3 g/cm3) due to lead content | Low (~2.23 g/cm3) |
| Refractive Index | High (~1.7-1.8); good optical clarity | Lower (~1.47) |
| Radiation Shielding | Excellent; effective for X-ray and gamma radiation | Poor; not suitable for radiation shielding |
| Durability | Good mechanical strength but less resistant to thermal stress | High durability; resistant to thermal and mechanical stress |
| Common Laboratory Uses | Radiation shielding windows, optical components | Beakers, flasks, tubing; standard laboratory glassware |
| Cost | Higher due to lead content and specialized uses | Lower; widely used and cost-effective |
Introduction to Laboratory Glassware Materials
Lead glass offers high density and excellent radiation shielding properties, making it suitable for specialized laboratory equipment requiring protection from X-rays and gamma rays. Borosilicate glass is prized for its superior thermal resistance, chemical durability, and low thermal expansion, making it the standard material for most laboratory glassware. Understanding the distinct physical and chemical properties of lead glass and borosilicate glass informs the selection of the most appropriate material for specific laboratory applications.
Overview of Lead Glass
Lead glass, characterized by its high lead oxide content ranging from 18% to 40%, offers exceptional clarity, high refractive index, and superior radiation shielding, making it ideal for specialized laboratory equipment like radiation shielding windows and protective barriers. Its density of approximately 3.1 to 3.3 g/cm3 provides enhanced durability against radiation exposure, whereas borosilicate glass, with a lower density around 2.23 g/cm3, is known for thermal resistance and chemical durability but lacks the same shielding capabilities. Lead glass excels in applications demanding both optical precision and protection from X-rays or gamma rays, distinguishing it from borosilicate glass commonly used for general chemical apparatus and heat-resistant containers.
Overview of Borosilicate Glass
Borosilicate glass is a type of laboratory glass known for its exceptional thermal resistance and chemical durability, making it ideal for handling high-temperature experiments and corrosive substances. It contains silica and boron trioxide, which provide low thermal expansion, reducing stress and minimizing the risk of breakage under rapid temperature changes. Compared to lead glass, borosilicate glass offers superior resistance to thermal shock, making it the preferred choice for laboratory equipment such as beakers, test tubes, and condensers.
Chemical Resistance: Lead Glass vs. Borosilicate Glass
Borosilicate glass exhibits superior chemical resistance compared to lead glass, making it ideal for handling strong acids, bases, and solvents in laboratory settings. Lead glass, containing higher lead oxide content, is more susceptible to chemical corrosion and leaching when exposed to harsh chemicals. The enhanced durability of borosilicate glass under aggressive chemical environments ensures longer equipment lifespan and reduced contamination risk.
Thermal Properties and Temperature Resistance
Lead glass offers excellent thermal insulation but has lower temperature resistance, typically withstanding up to 250degC, making it less suitable for high-heat laboratory applications. Borosilicate glass, renowned for its superior thermal shock resistance and stability, endures temperatures up to 500degC, supporting rapid temperature changes without cracking. The choice between lead glass and borosilicate glass in laboratory equipment depends on the thermal demands, with borosilicate preferred for tasks involving high or fluctuating temperatures.
Optical Clarity and Light Transmission Comparison
Lead glass exhibits superior optical clarity due to its higher refractive index, allowing for enhanced light transmission and reduced distortion in laboratory optics. Borosilicate glass, while more chemically resistant and thermally stable, offers slightly lower light transmission and a lower refractive index, resulting in less brilliance compared to lead glass. This makes lead glass preferable for applications requiring precise light manipulation, whereas borosilicate glass is favored for chemical durability and thermal shock resistance.
Mechanical Strength and Durability
Borosilicate glass exhibits superior mechanical strength and durability compared to lead glass, making it more resistant to thermal shock and mechanical stress in laboratory settings. Its low coefficient of thermal expansion (approximately 3.3 x 10-6 /degC) ensures enhanced resistance to cracking during rapid temperature changes. Lead glass, while offering higher density and optical clarity, is more prone to mechanical damage and less suitable for rigorous laboratory applications demanding robustness and longevity.
Safety Considerations and Toxicity
Lead glass contains lead oxide, which poses significant safety risks due to its potential toxicity and harmful effects when exposed to acids or high temperatures, requiring careful handling and disposal protocols in laboratories. Borosilicate glass, composed mainly of silica and boron trioxide, offers superior chemical resistance and thermal stability with negligible toxicity, making it a safer choice for laboratory equipment. Proper safety considerations prioritize borosilicate glass to minimize exposure to hazardous materials and ensure compliance with health and environmental regulations.
Applications in Laboratory Environments
Lead glass offers superior radiation shielding, making it ideal for laboratory environments dealing with X-rays or gamma rays in medical imaging and nuclear research. Borosilicate glass provides excellent thermal resistance and chemical durability, widely used in laboratories for heating, mixing, and handling corrosive substances. The choice between lead glass and borosilicate glass depends on specific application requirements such as radiation protection versus thermal and chemical resilience.
Cost Analysis and Availability
Lead glass typically costs more than borosilicate glass due to its higher density and lead content, impacting production expenses and pricing for laboratory equipment. Borosilicate glass is widely available and favored for laboratory use because of its excellent thermal resistance and chemical durability, making it a cost-effective option for most applications. The availability of borosilicate glass in multiple grades and forms contributes to lower procurement costs and faster supply chains compared to the less common, specialty lead glass.
Infographic: Lead glass vs Borosilicate glass for Laboratory equipment
 azmater.com
azmater.com