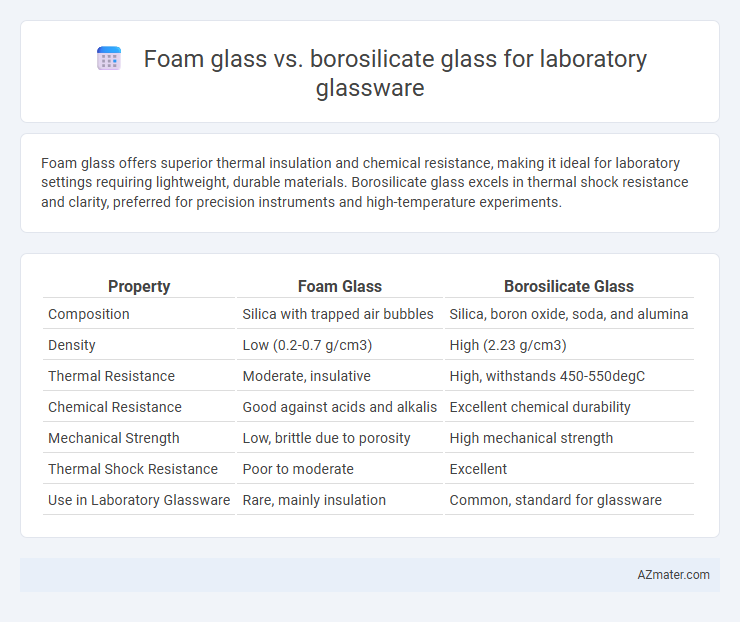
Foam glass offers superior thermal insulation and chemical resistance, making it ideal for laboratory settings requiring lightweight, durable materials. Borosilicate glass excels in thermal shock resistance and clarity, preferred for precision instruments and high-temperature experiments.
Table of Comparison
| Property | Foam Glass | Borosilicate Glass |
|---|---|---|
| Composition | Silica with trapped air bubbles | Silica, boron oxide, soda, and alumina |
| Density | Low (0.2-0.7 g/cm3) | High (2.23 g/cm3) |
| Thermal Resistance | Moderate, insulative | High, withstands 450-550degC |
| Chemical Resistance | Good against acids and alkalis | Excellent chemical durability |
| Mechanical Strength | Low, brittle due to porosity | High mechanical strength |
| Thermal Shock Resistance | Poor to moderate | Excellent |
| Use in Laboratory Glassware | Rare, mainly insulation | Common, standard for glassware |
Introduction to Laboratory Glassware Materials
Foam glass and borosilicate glass serve distinct roles in laboratory glassware due to their unique properties. Borosilicate glass is prized for its thermal resistance, chemical durability, and low thermal expansion, making it the standard material for most lab containers, beakers, and test tubes. Foam glass, composed of cellular glass with insulating properties, is typically used for specialized applications requiring thermal insulation or lightweight, rigid structures rather than direct chemical exposure.
What is Foam Glass?
Foam glass is a lightweight, porous material made by foaming molten glass, creating a cellular structure ideal for insulation and thermal resistance in laboratory settings. Unlike borosilicate glass, which is valued for its chemical durability and thermal shock resistance, foam glass offers superior insulation properties and mechanical cushioning. Its closed-cell structure provides excellent resistance to moisture, making it suitable for specialized applications where thermal insulation and lightweight properties are critical.
What is Borosilicate Glass?
Borosilicate glass is a type of laboratory glassware known for its exceptional thermal resistance and chemical durability, containing silica and boron trioxide as primary components. Unlike foam glass, which is porous and used mainly for insulation, borosilicate glass can withstand rapid temperature changes and exposure to corrosive chemicals, making it ideal for lab applications. This glass type offers superior strength and thermal shock resistance, crucial for reliable laboratory experiments and procedures.
Chemical Resistance Comparison
Foam glass exhibits excellent chemical resistance, particularly against acids, alkalis, and solvents due to its inert silicon dioxide structure and closed-cell foam composition, making it highly durable in aggressive chemical environments. Borosilicate glass also offers superior chemical resistance, especially to thermal shocks and corrosive chemicals like hydrochloric acid and sodium hydroxide, attributed to its boron oxide content that enhances its durability and stability. However, while borosilicate glass is preferred for laboratory glassware due to its resistance to thermal stress and chemical attack, foam glass is less common in labs but favored in insulation applications where chemical inertness and lightweight properties are crucial.
Thermal Resistance & Stability
Foam glass exhibits excellent thermal resistance with a low thermal conductivity around 0.1 W/m*K, making it highly effective for insulation in laboratory applications, but it is more brittle and less resistant to thermal shock compared to borosilicate glass. Borosilicate glass offers superior thermal stability, withstanding rapid temperature changes up to approximately 500degC without cracking due to its low coefficient of thermal expansion (about 3.3 x 10-6 /degC). While foam glass is ideal for insulation and structural support, borosilicate glass remains the preferred material for laboratory glassware requiring high thermal shock resistance and chemical durability under extreme thermal cycling.
Durability and Mechanical Strength
Foam glass offers enhanced mechanical strength and impact resistance due to its cellular structure, making it highly durable for laboratory applications requiring thermal insulation and shock absorption. Borosilicate glass, renowned for its exceptional chemical resistance and thermal shock tolerance, provides superior durability under extreme temperature fluctuations and corrosive environments. While foam glass excels in insulation and mechanical toughness, borosilicate glass remains the preferred material for laboratory glassware demanding high resistance to mechanical stress and chemical attack.
Safety Considerations in Laboratory Settings
Foam glass offers exceptional thermal insulation and chemical resistance, reducing the risk of thermal shock and corrosion in laboratory glassware applications. Borosilicate glass is highly resistant to thermal stress and chemical attack, making it a reliable choice for handling aggressive reagents and rapid temperature changes. Safety considerations favor borosilicate glass when thermal impact resistance is critical, while foam glass is preferred for insulating properties and minimizing heat transfer hazards.
Cost Analysis and Availability
Foam glass offers a cost-effective option for laboratory insulation due to its lower production costs compared to borosilicate glass, which involves more complex manufacturing processes. Borosilicate glass, valued for its thermal resistance and chemical durability, tends to have higher upfront costs but is widely available through established suppliers specialized in lab-grade materials. Availability of foam glass in laboratory settings is limited, often requiring custom orders, whereas borosilicate glass is readily accessible globally, ensuring faster procurement and consistent quality for laboratory glassware applications.
Application Suitability: Foam Glass vs Borosilicate Glass
Foam glass is primarily used for insulation and structural applications due to its lightweight and thermal resistance, but it lacks the chemical resistance and thermal shock durability required for laboratory glassware. Borosilicate glass excels in laboratory settings because of its high resistance to thermal shock, chemical corrosion, and mechanical stress, making it suitable for a wide range of chemical reactions and heating processes. The superior application suitability of borosilicate glass over foam glass in laboratory glassware ensures reliability and safety during precise scientific experiments.
Conclusion: Choosing the Right Laboratory Glassware Material
Foam glass offers excellent thermal insulation and chemical resistance, making it suitable for applications requiring heat retention and durability against corrosive substances. Borosilicate glass excels in thermal shock resistance and clarity, ideal for precise laboratory experiments involving rapid temperature changes and visual monitoring. Selecting between foam glass and borosilicate depends on the specific laboratory needs, prioritizing thermal properties, chemical resistance, and mechanical strength to ensure optimal performance and safety.
Infographic: Foam glass vs Borosilicate glass for Laboratory glassware
 azmater.com
azmater.com