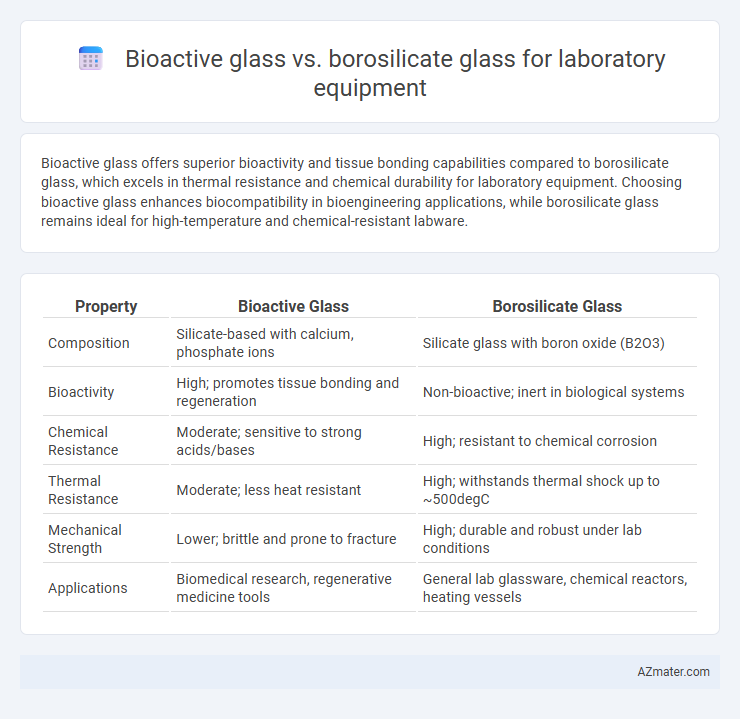
Bioactive glass offers superior bioactivity and tissue bonding capabilities compared to borosilicate glass, which excels in thermal resistance and chemical durability for laboratory equipment. Choosing bioactive glass enhances biocompatibility in bioengineering applications, while borosilicate glass remains ideal for high-temperature and chemical-resistant labware.
Table of Comparison
| Property | Bioactive Glass | Borosilicate Glass |
|---|---|---|
| Composition | Silicate-based with calcium, phosphate ions | Silicate glass with boron oxide (B2O3) |
| Bioactivity | High; promotes tissue bonding and regeneration | Non-bioactive; inert in biological systems |
| Chemical Resistance | Moderate; sensitive to strong acids/bases | High; resistant to chemical corrosion |
| Thermal Resistance | Moderate; less heat resistant | High; withstands thermal shock up to ~500degC |
| Mechanical Strength | Lower; brittle and prone to fracture | High; durable and robust under lab conditions |
| Applications | Biomedical research, regenerative medicine tools | General lab glassware, chemical reactors, heating vessels |
Introduction to Bioactive and Borosilicate Glass
Bioactive glass is a silica-based material known for its ability to bond with bone and stimulate tissue regeneration, making it ideal for biomedical applications. Borosilicate glass, composed primarily of silica and boron trioxide, offers exceptional thermal resistance and chemical durability, widely used in laboratory glassware for precise and consistent performance. Both materials serve distinct roles in laboratories: bioactive glass focuses on biological interactions, while borosilicate glass emphasizes structural integrity under heat and chemical stress.
Chemical Composition Differences
Bioactive glass primarily consists of silica (SiO2), calcium oxide (CaO), sodium oxide (Na2O), and phosphorus pentoxide (P2O5), designed to interact biologically by releasing ions that promote tissue regeneration. Borosilicate glass contains a high percentage of silica (around 80%) combined with boron oxide (B2O3), sodium oxide (Na2O), and aluminum oxide (Al2O3), offering superior thermal and chemical resistance for laboratory applications. The significant difference lies in borosilicate's boron oxide providing thermal stability, while bioactive glass's calcium and phosphorus components enable bioactivity and ion exchange with biological environments.
Physical and Mechanical Properties
Bioactive glass exhibits higher bioactivity and surface reactivity compared to borosilicate glass, making it ideal for applications requiring tissue integration and bonding. Borosilicate glass offers superior thermal resistance, chemical durability, and mechanical strength, with a low coefficient of thermal expansion around 3.3 x 10^-6 /degC, ensuring excellent dimensional stability under laboratory temperature fluctuations. The mechanical toughness of borosilicate glass surpasses that of bioactive glass, which tends to be more brittle and less resistant to impact or mechanical stress in standard laboratory settings.
Biocompatibility and Bioactivity
Bioactive glass exhibits superior biocompatibility and bioactivity compared to borosilicate glass, promoting cell attachment and tissue regeneration due to its ability to bond with bone and soft tissues. Borosilicate glass, while chemically resistant and thermally stable, lacks inherent bioactivity and does not support cellular interactions crucial for biomedical applications. The enhanced bioactive properties of bioactive glass make it the preferred choice for laboratory equipment involved in biological and medical research requiring direct contact with living tissues.
Thermal and Chemical Stability
Bioactive glass exhibits lower thermal stability compared to borosilicate glass, often softening at temperatures around 500degC, whereas borosilicate glass maintains structural integrity up to approximately 820degC due to its high thermal resistance. Chemically, borosilicate glass offers superior resistance to acids, alkalis, and solvents, making it ideal for rigorous laboratory conditions, while bioactive glass, designed for reactivity in biological environments, tends to degrade or leach ions when exposed to aqueous solutions. The enhanced chemical durability and thermal shock resistance of borosilicate glass ensure greater longevity and reliability for laboratory equipment under diverse experimental protocols.
Resistance to Laboratory Chemicals
Bioactive glass demonstrates moderate chemical resistance, reacting with strong acids and bases commonly found in laboratory settings, which can limit its durability under aggressive chemical exposure. Borosilicate glass offers superior resistance to a wide range of laboratory chemicals, including acids, bases, and solvents, making it the preferred choice for labware requiring consistent performance and longevity. The enhanced chemical stability of borosilicate glass results from its low coefficient of thermal expansion and durable silicate structure, ensuring minimal degradation during repeated use.
Applications in Laboratory Equipment
Bioactive glass is primarily used in laboratory equipment for applications requiring bioactivity and tissue integration, such as in biocompatible implants and regenerative medicine research tools. Borosilicate glass, with its high thermal resistance and chemical durability, is ideal for laboratory glassware like beakers, flasks, and test tubes that undergo frequent heating and exposure to corrosive chemicals. The choice between these materials depends on the specific laboratory application, with bioactive glass suited for biomedical experiments and borosilicate glass preferred for general chemical and thermal stability in laboratory settings.
Cost Comparison and Availability
Bioactive glass, primarily used for medical and dental applications, tends to be more expensive than borosilicate glass due to its specialized composition and bioactive properties. Borosilicate glass, widely available and cost-effective, is preferred for general laboratory equipment because of its excellent thermal resistance and chemical durability. The higher production costs and limited suppliers of bioactive glass result in lower availability, while borosilicate glass features extensive market distribution and competitive pricing.
Environmental Impact and Sustainability
Bioactive glass offers superior environmental benefits compared to borosilicate glass due to its bioresorbable properties, allowing it to break down naturally without leaving harmful residues. Borosilicate glass, while chemically resistant and durable for laboratory use, requires significant energy for production and recycling, contributing to a larger carbon footprint. Sustainable laboratory practices favor bioactive glass in applications where biodegradability and reduced waste are critical, supporting greener lab environments.
Conclusion: Choosing the Right Glass for Laboratory Use
Bioactive glass offers excellent biocompatibility and chemical durability, making it ideal for applications involving biological interactions and cell culture. Borosilicate glass provides superior thermal shock resistance and chemical stability, making it preferred for general laboratory equipment such as flasks and beakers exposed to rapid temperature changes. Selecting the right glass depends on specific laboratory requirements, balancing the need for biological compatibility versus thermal and chemical resilience.
Infographic: Bioactive glass vs Borosilicate glass for Laboratory equipment
 azmater.com
azmater.com