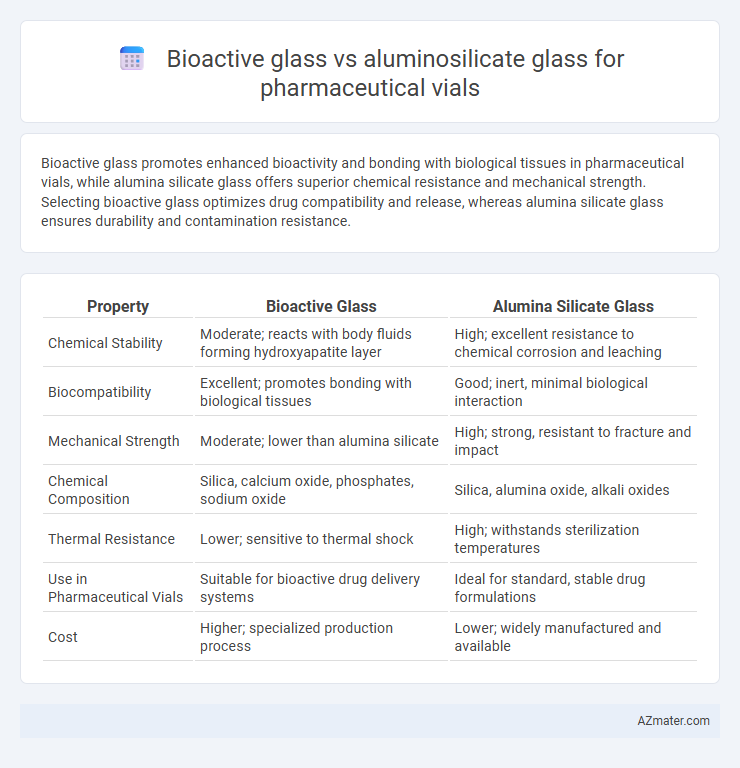
Bioactive glass promotes enhanced bioactivity and bonding with biological tissues in pharmaceutical vials, while alumina silicate glass offers superior chemical resistance and mechanical strength. Selecting bioactive glass optimizes drug compatibility and release, whereas alumina silicate glass ensures durability and contamination resistance.
Table of Comparison
| Property | Bioactive Glass | Alumina Silicate Glass |
|---|---|---|
| Chemical Stability | Moderate; reacts with body fluids forming hydroxyapatite layer | High; excellent resistance to chemical corrosion and leaching |
| Biocompatibility | Excellent; promotes bonding with biological tissues | Good; inert, minimal biological interaction |
| Mechanical Strength | Moderate; lower than alumina silicate | High; strong, resistant to fracture and impact |
| Chemical Composition | Silica, calcium oxide, phosphates, sodium oxide | Silica, alumina oxide, alkali oxides |
| Thermal Resistance | Lower; sensitive to thermal shock | High; withstands sterilization temperatures |
| Use in Pharmaceutical Vials | Suitable for bioactive drug delivery systems | Ideal for standard, stable drug formulations |
| Cost | Higher; specialized production process | Lower; widely manufactured and available |
Introduction to Glass Types in Pharmaceutical Packaging
Bioactive glass and alumina silicate glass serve distinct roles in pharmaceutical vial applications, with bioactive glass known for its ability to bond with biological tissues, enhancing drug delivery systems. Alumina silicate glass is prized for its high chemical durability and mechanical strength, providing robust protection against contamination and physical damage. Choosing the appropriate glass type impacts drug stability, vial integrity, and overall packaging performance in pharmaceutical environments.
Overview of Bioactive Glass Composition and Properties
Bioactive glass in pharmaceutical vials primarily consists of silica (SiO2), calcium oxide (CaO), sodium oxide (Na2O), and phosphorus pentoxide (P2O5), which together create a highly reactive surface that promotes bone bonding and biointegration. Its unique properties include high biocompatibility, controlled degradation rates, and the ability to release therapeutic ions that can enhance drug stability and efficacy. Compared to alumina silicate glass, bioactive glass offers enhanced chemical durability, improved bioactivity, and tailored dissolution profiles critical for advanced pharmaceutical applications.
Alumina Silicate Glass: Structure and Key Features
Alumina silicate glass used in pharmaceutical vials features a robust amorphous structure incorporating aluminum oxide (Al2O3) and silicon dioxide (SiO2), providing enhanced chemical durability and mechanical strength. Its high resistance to thermal shock and low ion leaching ensure drug stability and extended shelf life. The alumina silicate matrix offers excellent barrier properties against moisture and contaminants, making it ideal for sterile pharmaceutical packaging.
Chemical Stability in Pharmaceutical Environments
Bioactive glass exhibits superior chemical stability in pharmaceutical environments due to its controlled ion release and resistance to hydrolytic degradation, ensuring minimal interaction with drug formulations. Alumina silicate glass offers high chemical resistance with excellent inertness to a wide range of pH conditions but may be more prone to surface corrosion under extreme alkaline or acidic pharmaceutical solutions. The choice between bioactive glass and alumina silicate glass hinges on compatibility with specific drug chemistries and long-term stability requirements in vial applications.
Mechanical Strength and Durability Comparison
Bioactive glass offers superior mechanical strength and durability compared to alumina silicate glass, making it highly suitable for pharmaceutical vials subjected to high-impact forces and thermal stress. Its unique composition enhances resistance to cracking and chemical degradation, extending the vial's lifespan in rigorous pharmaceutical environments. Alumina silicate glass, while chemically stable, generally exhibits lower fracture toughness and reduced durability under sterilization processes compared to bioactive glass.
Interaction with Pharmaceutical Compounds
Bioactive glass exhibits controlled ion release that can influence drug stability and enhance bioactivity, making it suitable for specific pharmaceutical formulations. Alumina silicate glass offers superior chemical resistance and minimal interaction with pharmaceutical compounds, ensuring drug integrity and reduced contamination risk. The choice between bioactive glass and alumina silicate glass depends on the desired balance between bioactivity and chemical inertness for the vial's intended pharmaceutical application.
Sterilization Compatibility and Resistance
Bioactive glass exhibits superior sterilization compatibility due to its chemical stability and resistance to thermal shock, making it ideal for repeated autoclave cycles used in pharmaceutical vial production. Alumina silicate glass offers high mechanical strength but may suffer from surface degradation or ion leaching when exposed to aggressive sterilization methods like gamma irradiation or ethylene oxide. The choice between bioactive glass and alumina silicate glass hinges on the required balance between chemical inertness and mechanical durability under sterilization conditions.
Cost Analysis: Bioactive vs Alumina Silicate Glass
Bioactive glass offers enhanced biocompatibility and bioactivity but comes at a higher production cost compared to alumina silicate glass, which is more cost-effective due to its established manufacturing processes and raw material availability. Alumina silicate glass provides excellent chemical durability and mechanical strength, making it a budget-friendly choice for large-scale pharmaceutical vial production. Cost analysis reveals that while bioactive glass ensures therapeutic benefits, alumina silicate glass remains the preferred option when minimizing expenses without compromising vial performance.
Regulatory Acceptance and Compliance
Bioactive glass and alumina silicate glass exhibit distinct profiles in regulatory acceptance and compliance for pharmaceutical vials, with alumina silicate glass benefiting from established approval owing to its widespread historical use and proven chemical inertness. Regulatory agencies such as the FDA and EMA recognize alumina silicate glass under USP <660> standards, ensuring compatibility with a broad range of pharmaceutical formulations and sterilization processes. Bioactive glass, while promising for its bioactivity and healing properties, faces stricter regulatory scrutiny due to its novel composition and potential interactions, necessitating extensive biocompatibility and stability data before achieving similar compliance benchmarks.
Future Trends in Pharmaceutical Vial Materials
Bioactive glass offers enhanced biocompatibility and antimicrobial properties, making it a promising candidate for next-generation pharmaceutical vials that demand improved sterility and drug stability. Alumina silicate glass remains valued for its superior chemical durability and mechanical strength, ensuring vials withstand rigorous sterilization and transportation processes. Future trends indicate increasing integration of bioactive glass coatings on alumina silicate substrates to combine biofunctionality with robust physical performance in pharmaceutical packaging.
Infographic: Bioactive glass vs Alumina silicate glass for Pharmaceutical vial
 azmater.com
azmater.com