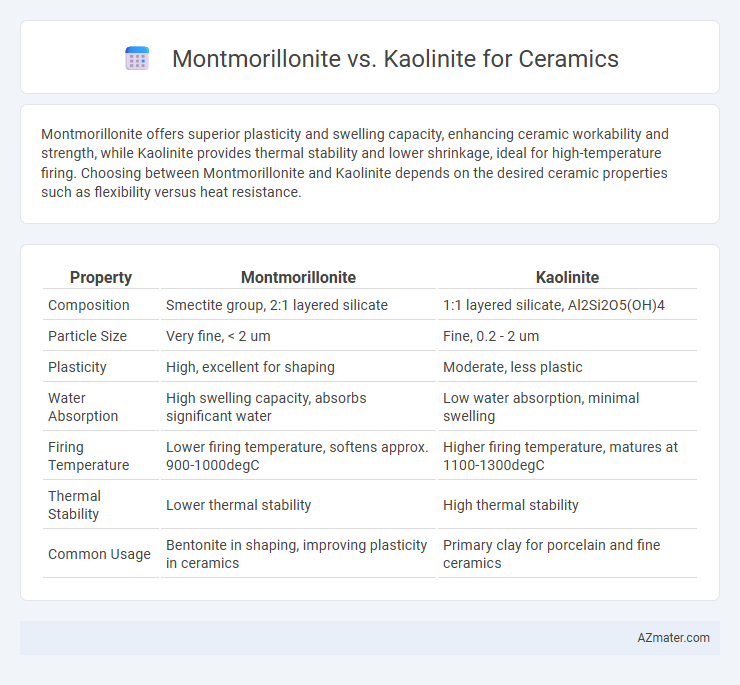
Montmorillonite offers superior plasticity and swelling capacity, enhancing ceramic workability and strength, while Kaolinite provides thermal stability and lower shrinkage, ideal for high-temperature firing. Choosing between Montmorillonite and Kaolinite depends on the desired ceramic properties such as flexibility versus heat resistance.
Table of Comparison
| Property | Montmorillonite | Kaolinite |
|---|---|---|
| Composition | Smectite group, 2:1 layered silicate | 1:1 layered silicate, Al2Si2O5(OH)4 |
| Particle Size | Very fine, < 2 um | Fine, 0.2 - 2 um |
| Plasticity | High, excellent for shaping | Moderate, less plastic |
| Water Absorption | High swelling capacity, absorbs significant water | Low water absorption, minimal swelling |
| Firing Temperature | Lower firing temperature, softens approx. 900-1000degC | Higher firing temperature, matures at 1100-1300degC |
| Thermal Stability | Lower thermal stability | High thermal stability |
| Common Usage | Bentonite in shaping, improving plasticity in ceramics | Primary clay for porcelain and fine ceramics |
Introduction to Montmorillonite and Kaolinite
Montmorillonite and Kaolinite are two essential clay minerals widely used in ceramics due to their distinct structural and chemical properties. Montmorillonite, a smectite group mineral, is characterized by its high cation exchange capacity and swelling behavior, making it valuable for improving plasticity and workability in ceramic formulations. Kaolinite, a layered silicate mineral with low shrink-swell capacity and excellent refractory properties, provides strength and whiteness to ceramic products, serving as a primary raw material in porcelain and fine china manufacturing.
Chemical Composition and Structure Comparison
Montmorillonite and kaolinite differ significantly in chemical composition and structure, impacting their ceramic applications. Montmorillonite has a 2:1 clay mineral structure with two tetrahedral silica sheets sandwiching an octahedral alumina sheet, exhibiting high cation exchange capacity due to its expandable layers and presence of magnesium and iron ions. Kaolinite features a 1:1 structure composed of one tetrahedral silica sheet and one octahedral alumina sheet, characterized by low shrink-swell capacity and lower cation exchange capacity, with a chemical formula primarily Al2Si2O5(OH)4, making it more stable and less plastic in ceramic processing.
Plasticity and Workability Differences
Montmorillonite exhibits significantly higher plasticity than kaolinite due to its expansive layered structure, allowing greater water absorption and flexibility during shaping. Kaolinite's lower plasticity results in reduced workability, making it more suitable for applications requiring firmness and minimal deformation. The superior plasticity of montmorillonite enhances its performance in ceramic processes demanding intricate molding and enhanced green strength.
Firing Temperatures and Behavior
Montmorillonite and kaolinite differ significantly in their firing temperatures and behavior in ceramic applications. Montmorillonite typically starts to sinter at lower temperatures around 900-1000degC, exhibiting high plasticity but prone to swelling and structural changes during firing. Kaolinite, with a higher firing temperature range of approximately 1000-1300degC, demonstrates better thermal stability and vitrification, resulting in denser, stronger ceramics with minimal deformation.
Impact on Ceramic Strength and Durability
Montmorillonite enhances ceramic strength and durability due to its high cation exchange capacity and swelling properties, allowing better particle bonding and stress absorption during firing. Kaolinite contributes to ceramic resilience by providing a stable aluminosilicate framework with low shrinkage and excellent whiteness, improving surface hardness and resistance to chemical attack. The balanced use of montmorillonite and kaolinite optimizes ceramic microstructure, resulting in improved mechanical properties and long-term durability.
Water Absorption and Shrinkage Rates
Montmorillonite exhibits higher water absorption due to its expansive layered structure, which significantly increases ceramic porosity compared to kaolinite. Kaolinite's lower water absorption results in reduced shrinkage rates during drying and firing, providing better dimensional stability in ceramic products. The swelling behavior of montmorillonite can cause excessive shrinkage and cracking, making kaolinite more favorable for producing durable ceramics with controlled water absorption and minimal shrinkage.
Influence on Glaze Interaction
Montmorillonite's high cation-exchange capacity enhances glaze adhesion and fluidity, promoting better interaction and smoother finishes in ceramic applications. Kaolinite's lower plasticity results in less reactive glaze surfaces, offering more controlled melting and distinct texture in the fired glaze. The choice between Montmorillonite and Kaolinite directly affects glaze absorption, fusion behavior, and the overall aesthetic quality of ceramic glazes.
Availability and Cost Factors
Montmorillonite, abundant in regions such as the United States and Brazil, often incurs higher extraction and processing costs due to its swelling properties and water retention, which require specialized treatment for ceramic production. Kaolinite is widely available globally, particularly in China, the UK, and the USA, and its relatively low mining and processing costs make it a more economical choice for ceramic manufacturers. The cost-effectiveness of kaolinite contributes to its preference in mass ceramic production, while montmorillonite's unique properties justify its use in specialized, higher-end applications despite higher costs.
Applications in Ceramic Art and Industry
Montmorillonite offers superior plasticity and swelling properties, making it ideal for ceramic art that requires intricate shaping and fine detailing. Kaolinite, known for its low shrinkage and high whiteness, is preferred in industrial ceramics for producing durable, smooth, and aesthetically clean surfaces. Both minerals play critical roles in formulations where Montmorillonite enhances workability while Kaolinite provides structural strength and fired whiteness.
Choosing the Right Clay for Your Project
Montmorillonite and Kaolinite are essential clay minerals for ceramics, with Montmorillonite offering high plasticity and excellent water retention, making it ideal for intricate shaping and molding. Kaolinite provides superior whiteness and firing strength, delivering a smooth texture and crisp finish suited for fine porcelain and durable pottery. Selecting the right clay depends on the project's requirements for workability, firing temperature, and final product aesthetics.
Infographic: Montmorillonite vs Kaolinite for Ceramic
 azmater.com
azmater.com