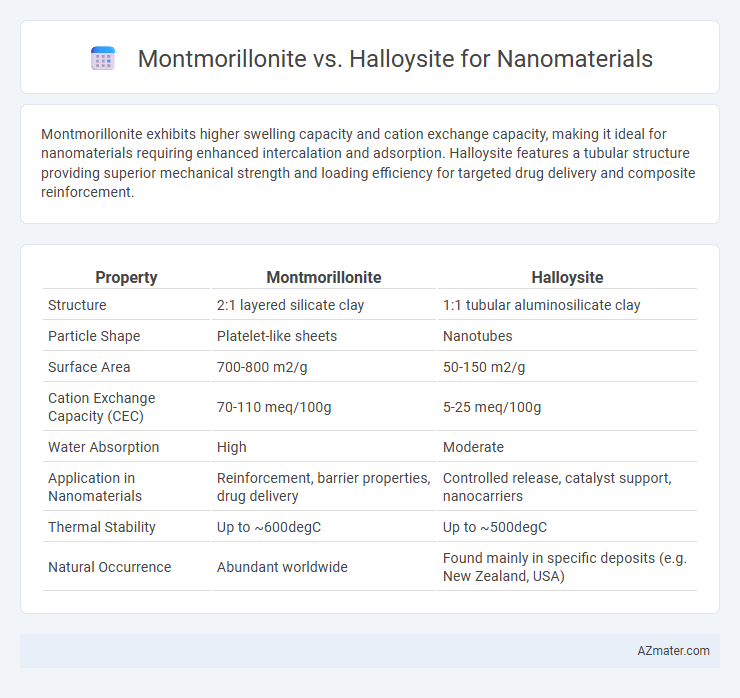
Montmorillonite exhibits higher swelling capacity and cation exchange capacity, making it ideal for nanomaterials requiring enhanced intercalation and adsorption. Halloysite features a tubular structure providing superior mechanical strength and loading efficiency for targeted drug delivery and composite reinforcement.
Table of Comparison
| Property | Montmorillonite | Halloysite |
|---|---|---|
| Structure | 2:1 layered silicate clay | 1:1 tubular aluminosilicate clay |
| Particle Shape | Platelet-like sheets | Nanotubes |
| Surface Area | 700-800 m2/g | 50-150 m2/g |
| Cation Exchange Capacity (CEC) | 70-110 meq/100g | 5-25 meq/100g |
| Water Absorption | High | Moderate |
| Application in Nanomaterials | Reinforcement, barrier properties, drug delivery | Controlled release, catalyst support, nanocarriers |
| Thermal Stability | Up to ~600degC | Up to ~500degC |
| Natural Occurrence | Abundant worldwide | Found mainly in specific deposits (e.g. New Zealand, USA) |
Introduction to Clay Minerals in Nanomaterials
Montmorillonite and halloysite are prominent clay minerals widely utilized as nanomaterials due to their unique layered silicate structures. Montmorillonite features a 2:1 phyllosilicate configuration with high cation exchange capacity and swelling properties, making it suitable for drug delivery and nanocomposites. Halloysite, with its naturally occurring tubular morphology, offers distinct advantages in encapsulation and controlled release applications within nanotechnology.
Overview of Montmorillonite and Halloysite
Montmorillonite and halloysite are clay minerals extensively utilized as nanomaterials due to their unique layered and tubular structures, respectively. Montmorillonite features a 2:1 phyllosilicate structure with high cation exchange capacity and swelling properties, making it ideal for adsorption and composite applications. Halloysite, characterized by its naturally occurring hollow nanotubes, offers superior surface area and biocompatibility, which are advantageous for drug delivery and ceramics in nanotechnology.
Chemical Structures: Montmorillonite vs Halloysite
Montmorillonite features a 2:1 layered structure composed of two tetrahedral silica sheets sandwiching an octahedral alumina sheet, which facilitates high cation exchange capacity and surface reactivity. Halloysite possesses a similar 1:1 kaolin-type structure with intimate alumina and silica layers, but it forms naturally as hollow nanotubes due to hydration-induced curvature. The distinctive tubular morphology of halloysite versus the platy morphology of montmorillonite critically influences their dispersion, surface area, and functionalization potential in nanomaterial applications.
Morphological Differences and Nanotube Formation
Montmorillonite exhibits a layered, plate-like morphology with high cation exchange capacity, making it effective for nanocomposite reinforcement, whereas Halloysite naturally forms tubular nanostructures with diameters typically ranging from 50 to 100 nm and lengths up to 1 um. The unique hollow nanotube morphology of Halloysite facilitates enhanced loading and controlled release of active agents compared to the flat, stacked layers of Montmorillonite. These morphological differences influence their surface area, adsorption properties, and intercalation capabilities, critical factors in nanomaterial design and functionality.
Surface Area and Porosity Comparison
Montmorillonite exhibits a higher surface area, typically ranging from 200 to 800 m2/g, which enhances its adsorption capacity compared to Halloysite's 60 to 100 m2/g. Halloysite nanotubes possess unique tubular porosity structures with mesopores around 2 to 50 nm, offering distinct nanocapsule potentials, while Montmorillonite's layered structure contributes to greater swelling and interlayer porosity. The choice between Montmorillonite and Halloysite for nanomaterials hinges on the desired balance between specific surface area and pore morphology for applications like catalysis, drug delivery, or environmental remediation.
Functionalization Capabilities for Nanotechnology
Montmorillonite exhibits superior cation exchange capacity and larger surface area compared to halloysite, enabling more versatile functionalization for nanomaterial applications. Halloysite nanotubes, with their unique tubular morphology and natural lumen, offer targeted drug delivery potentials and ease of surface modification via inner lumen functionalization. Both nanoclays provide distinct functionalization pathways, with montmorillonite favoring layered intercalation chemistry while halloysite excels in lumen encapsulation and selective surface grafting.
Mechanical and Thermal Properties
Montmorillonite exhibits superior mechanical reinforcement due to its high aspect ratio and layered silicate structure, enhancing stiffness and tensile strength in nanocomposites. Halloysite, with its tubular morphology, offers improved thermal stability and heat resistance, making it suitable for applications requiring enhanced thermal insulation. Both nanomaterials contribute uniquely to the mechanical and thermal performance of composites, with Montmorillonite favoring mechanical enhancement and Halloysite excelling in thermal properties.
Applications in Drug Delivery and Biomedicine
Montmorillonite exhibits high cation-exchange capacity and swelling properties, making it effective for controlled drug release and targeted delivery systems in biomedicine. Halloysite's tubular nanostructure offers unique encapsulation abilities, enhancing drug loading efficiency and sustained release profiles for therapeutic applications. Both nanoclays contribute to biocompatibility and reduced toxicity in drug delivery platforms, but halloysite's morphology provides superior versatility in functionalization and biomedical device integration.
Environmental and Industrial Uses
Montmorillonite and halloysite are both clay minerals widely used as nanomaterials due to their high surface area and unique structural properties, with montmorillonite possessing a layered structure ideal for adsorption and catalyst support in environmental remediation. Halloysite nanotubes, featuring a hollow tubular morphology, excel in controlled release applications and pollutant encapsulation in water treatment systems. Industrially, montmorillonite is favored for its swelling capacity in drilling fluids and polymer nanocomposites, whereas halloysite's tubular form enhances performance in cosmetic formulations and as nanocarriers for corrosion inhibitors.
Future Trends in Montmorillonite and Halloysite Nanomaterials
Montmorillonite and halloysite nanomaterials demonstrate distinct advantages in nanocomposite and drug delivery applications due to their layered and tubular structures, respectively. Future trends emphasize the functionalization of montmorillonite to enhance its adsorption capacity and thermal stability, while research on halloysite focuses on controlled release mechanisms and bioactive molecule encapsulation. Emerging applications include environmental remediation, catalysis, and biomedicine, propelled by advances in surface modification and hybrid nanomaterial synthesis.
Infographic: Montmorillonite vs Halloysite for Nanomaterial
 azmater.com
azmater.com