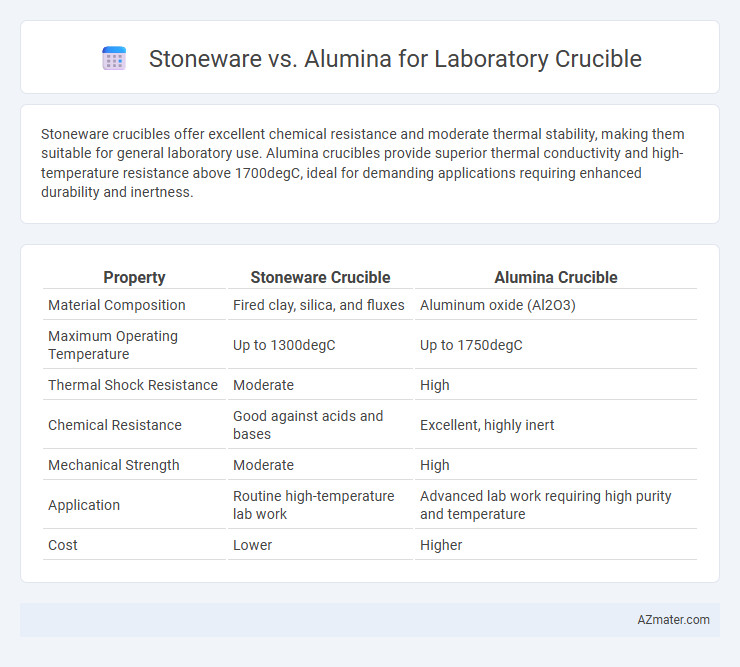
Stoneware crucibles offer excellent chemical resistance and moderate thermal stability, making them suitable for general laboratory use. Alumina crucibles provide superior thermal conductivity and high-temperature resistance above 1700degC, ideal for demanding applications requiring enhanced durability and inertness.
Table of Comparison
| Property | Stoneware Crucible | Alumina Crucible |
|---|---|---|
| Material Composition | Fired clay, silica, and fluxes | Aluminum oxide (Al2O3) |
| Maximum Operating Temperature | Up to 1300degC | Up to 1750degC |
| Thermal Shock Resistance | Moderate | High |
| Chemical Resistance | Good against acids and bases | Excellent, highly inert |
| Mechanical Strength | Moderate | High |
| Application | Routine high-temperature lab work | Advanced lab work requiring high purity and temperature |
| Cost | Lower | Higher |
Introduction to Laboratory Crucibles
Laboratory crucibles are essential tools designed to withstand high temperatures for melting, calcining, and combustion processes in scientific research. Stoneware crucibles, made from dense, refractory clay, offer excellent thermal shock resistance and chemical durability, making them ideal for general laboratory use. Alumina crucibles provide superior mechanical strength and higher melting points, suitable for experiments requiring intense heat and corrosive substances.
Understanding Stoneware Crucibles
Stoneware crucibles offer exceptional chemical resistance and thermal stability, making them ideal for high-temperature laboratory applications involving non-corrosive materials. Their dense, vitrified composition ensures durability and minimizes porosity, reducing contamination risk during heating processes. Unlike alumina crucibles, stoneware provides cost-effective performance but has lower thermal conductivity and mechanical strength.
Key Properties of Alumina Crucibles
Alumina crucibles for laboratory use offer superior thermal stability, with melting points above 2072degC, making them ideal for high-temperature applications. Their excellent chemical inertness resists corrosion and contamination from aggressive acids, alkalis, and molten metals, ensuring sample integrity. High mechanical strength and low thermal expansion reduce cracking risk during rapid heating and cooling compared to stoneware crucibles.
Thermal Resistance: Stoneware vs Alumina
Stoneware crucibles typically withstand temperatures up to 1200degC, making them suitable for moderate thermal applications, whereas alumina crucibles endure extreme heat up to 1750degC or higher, ideal for high-temperature laboratory processes. Alumina's superior thermal resistance ensures minimal thermal expansion and higher mechanical strength, reducing the risk of cracking during rapid temperature changes. Stoneware is more cost-effective but less durable under intense thermal stress compared to the robust, high-purity alumina crucibles commonly used in advanced material synthesis and metallurgical analysis.
Chemical Compatibility and Inertness
Stoneware crucibles offer excellent chemical resistance to many acids, alkalis, and salts but may react with strong alkaline materials at high temperatures, limiting their inertness in certain chemical environments. Alumina crucibles provide superior chemical inertness and withstand aggressive corrosive substances, making them ideal for high-purity applications and procedures involving molten metals or slags. The high alumina content ensures minimal contamination risk and excellent resistance to thermal shock during repeated heating cycles in laboratory settings.
Mechanical Strength and Durability
Stoneware crucibles offer moderate mechanical strength and good thermal shock resistance, making them suitable for general laboratory heating tasks. Alumina crucibles exhibit superior mechanical strength and exceptional durability, with high resistance to abrasion, corrosion, and high temperatures up to 1750degC. The enhanced toughness and chemical stability of alumina make it ideal for demanding applications requiring repeated use and exposure to aggressive materials.
Cost Comparison and Availability
Stoneware laboratory crucibles generally offer a lower cost and higher availability due to widespread manufacturing and commonly used raw materials. Alumina crucibles, on the other hand, carry a higher price because of their superior thermal resistance and durability, yet they can be less readily available in some regions. The choice between stoneware and alumina often balances budget constraints against performance needs and regional supply conditions.
Typical Applications in Laboratories
Stoneware crucibles are commonly used for general heating and ashing of soil, minerals, and chemicals due to their excellent resistance to thermal shock and moderate chemical durability. Alumina crucibles offer superior performance in high-temperature applications, such as melting metals, conducting precise chemical reactions, and handling corrosive substances, thanks to their exceptional thermal stability and chemical inertness. Both materials serve critical roles in laboratory settings, with stoneware favored for routine, lower temperature tasks and alumina preferred for demanding, high-temperature processes.
Cleaning and Maintenance Requirements
Stoneware crucibles require regular cleaning with mild detergents to prevent residue buildup due to their porous nature, making them susceptible to staining and contamination if not properly maintained. Alumina crucibles, offering higher chemical resistance and non-porous surfaces, allow for easier cleaning and minimal maintenance, reducing the risk of cross-contamination in laboratory applications. The durability of alumina facilitates aggressive cleaning methods like acid washes without damage, whereas stoneware demands gentle handling to preserve integrity.
How to Select the Right Crucible for Your Needs
Choosing between stoneware and alumina crucibles depends on the specific thermal and chemical requirements of your laboratory processes. Stoneware crucibles offer excellent resistance to moderate temperatures and chemical corrosion, ideal for organic material combustion and low-temperature ashing. Alumina crucibles provide superior thermal stability, higher melting points above 1700degC, and exceptional resistance to acidic and basic slags, making them suitable for high-temperature fusion and metal analysis.
Infographic: Stoneware vs Alumina for Laboratory Crucible
 azmater.com
azmater.com