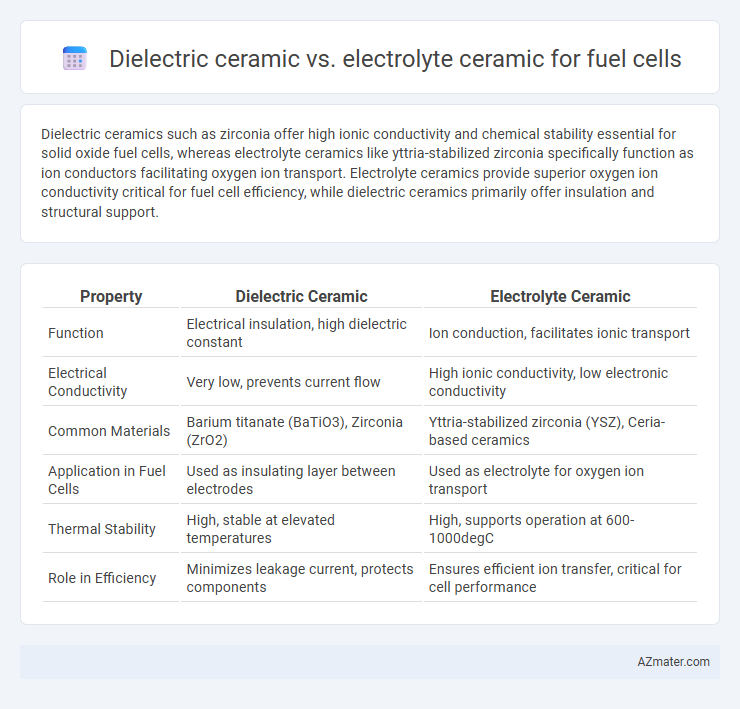
Dielectric ceramics such as zirconia offer high ionic conductivity and chemical stability essential for solid oxide fuel cells, whereas electrolyte ceramics like yttria-stabilized zirconia specifically function as ion conductors facilitating oxygen ion transport. Electrolyte ceramics provide superior oxygen ion conductivity critical for fuel cell efficiency, while dielectric ceramics primarily offer insulation and structural support.
Table of Comparison
| Property | Dielectric Ceramic | Electrolyte Ceramic |
|---|---|---|
| Function | Electrical insulation, high dielectric constant | Ion conduction, facilitates ionic transport |
| Electrical Conductivity | Very low, prevents current flow | High ionic conductivity, low electronic conductivity |
| Common Materials | Barium titanate (BaTiO3), Zirconia (ZrO2) | Yttria-stabilized zirconia (YSZ), Ceria-based ceramics |
| Application in Fuel Cells | Used as insulating layer between electrodes | Used as electrolyte for oxygen ion transport |
| Thermal Stability | High, stable at elevated temperatures | High, supports operation at 600-1000degC |
| Role in Efficiency | Minimizes leakage current, protects components | Ensures efficient ion transfer, critical for cell performance |
Introduction to Ceramic Materials in Fuel Cells
Dielectric ceramics in fuel cells provide high electrical insulation and chemical stability, making them ideal for separating electrodes and preventing short circuits. Electrolyte ceramics, such as yttria-stabilized zirconia (YSZ), enable oxygen ion conduction at elevated temperatures, which is critical for efficient fuel cell operation. The choice between dielectric and electrolyte ceramics directly impacts fuel cell performance, durability, and operating temperature range.
Overview of Dielectric Ceramics
Dielectric ceramics in fuel cells serve as insulating materials with high dielectric strength and low electrical conductivity, crucial for separating electrodes and preventing electrical short circuits. These ceramics typically exhibit excellent thermal stability and mechanical strength, enhancing fuel cell durability under high-temperature operating conditions. Compared to electrolyte ceramics, which primarily conduct ions, dielectric ceramics focus on electrical insulation and structural support within the fuel cell assembly.
Overview of Electrolyte Ceramics
Electrolyte ceramics in fuel cells primarily serve as ion-conducting membranes, enabling oxygen ion transport between electrodes while preventing gas crossover, crucial for efficient solid oxide fuel cell (SOFC) operation. Common electrolyte materials include yttria-stabilized zirconia (YSZ) and gadolinium-doped ceria (GDC), valued for their high ionic conductivity and chemical stability at elevated temperatures. The performance of electrolyte ceramics directly influences fuel cell efficiency, durability, and operating temperature range, making material selection vital for optimizing SOFC technology.
Key Properties: Dielectric vs Electrolyte Ceramics
Dielectric ceramics, such as barium titanate and zirconia-based materials, exhibit high electrical insulation and strong polarization capabilities, making them ideal for capacitive applications but unsuitable for ionic conduction in fuel cells. Electrolyte ceramics, predominantly yttria-stabilized zirconia (YSZ) and ceria-based materials, offer exceptional ionic conductivity and chemical stability at elevated temperatures, enabling efficient oxygen ion transport essential for solid oxide fuel cells (SOFCs). Key properties like dielectric constant, ionic conductivity, and thermal stability distinctly separate dielectric ceramics, which prevent current flow, from electrolyte ceramics that facilitate ionic movement within fuel cell architectures.
Role of Dielectric Ceramics in Fuel Cells
Dielectric ceramics in fuel cells serve as crucial insulating materials that prevent electron flow while allowing ion transport, enhancing overall efficiency by maintaining charge separation between electrodes. Their high dielectric constant improves the electrochemical performance by stabilizing the electric field, which supports ion conductivity without electronic conduction. Unlike electrolyte ceramics that primarily conduct ions, dielectric ceramics contribute to fuel cell durability and efficiency by minimizing electrical losses and ensuring stable operation under various temperature conditions.
Role of Electrolyte Ceramics in Fuel Cells
Electrolyte ceramics in fuel cells are crucial for conducting oxygen ions from the cathode to the anode, enabling the electrochemical reaction that generates electricity. These ceramics, often made from materials like yttria-stabilized zirconia (YSZ), provide high ionic conductivity and chemical stability at elevated temperatures. Unlike dielectric ceramics, which primarily serve as insulators, electrolyte ceramics facilitate ion transport, directly impacting fuel cell efficiency and performance.
Performance Comparison: Efficiency and Conductivity
Dielectric ceramic materials in fuel cells exhibit high electrical insulation but low ionic conductivity, resulting in limited efficiency for ion transport compared to electrolyte ceramics. Electrolyte ceramics, such as yttria-stabilized zirconia (YSZ), demonstrate superior ionic conductivity and enable enhanced electrochemical performance by facilitating efficient oxygen ion transport. The higher ionic conductivity of electrolyte ceramics directly contributes to improved fuel cell efficiency and overall power output.
Material Stability and Durability in Fuel Cell Environments
Dielectric ceramics, such as zirconia-based materials, exhibit high chemical stability and resist degradation in the oxidative and high-temperature environments of solid oxide fuel cells (SOFCs), ensuring long-term durability. Electrolyte ceramics, primarily yttria-stabilized zirconia (YSZ) or doped ceria, maintain ionic conductivity under reducing and oxidizing atmospheres but may experience phase instability or grain growth affecting durability over extended operation. Material selection for fuel cells prioritizes dielectric ceramics for insulating components due to their stability, while electrolyte ceramics require optimized doping and microstructure control to balance ionic conductivity with mechanical and chemical durability.
Application Suitability: When to Use Dielectric or Electrolyte Ceramics
Dielectric ceramics are suitable for applications requiring high electrical insulation and stability under thermal stress, often used as insulating layers in solid oxide fuel cells (SOFCs) to prevent undesired electron flow. Electrolyte ceramics, such as yttria-stabilized zirconia (YSZ), are essential in fuel cells for efficient oxygen ion conduction, enabling the electrochemical reaction necessary for energy generation. Choosing between dielectric and electrolyte ceramics depends on whether the component's primary function is electrical insulation or ionic conduction within the fuel cell architecture.
Future Trends and Innovations in Ceramic Fuel Cell Materials
Dielectric ceramic materials in fuel cells offer high ionic conductivity and excellent thermal stability, enabling improved efficiency and durability in solid oxide fuel cells (SOFCs). Electrolyte ceramics continue to evolve with innovations like doped ceria and bismuth oxide-based electrolytes, which enhance ionic transport at lower operating temperatures, reducing degradation. Future trends emphasize nano-engineering and composite ceramics to optimize ionic conductivity and mechanical robustness, pushing fuel cell technology toward more sustainable and cost-effective energy solutions.
Infographic: Dielectric ceramic vs Electrolyte ceramic for Fuel cell
 azmater.com
azmater.com