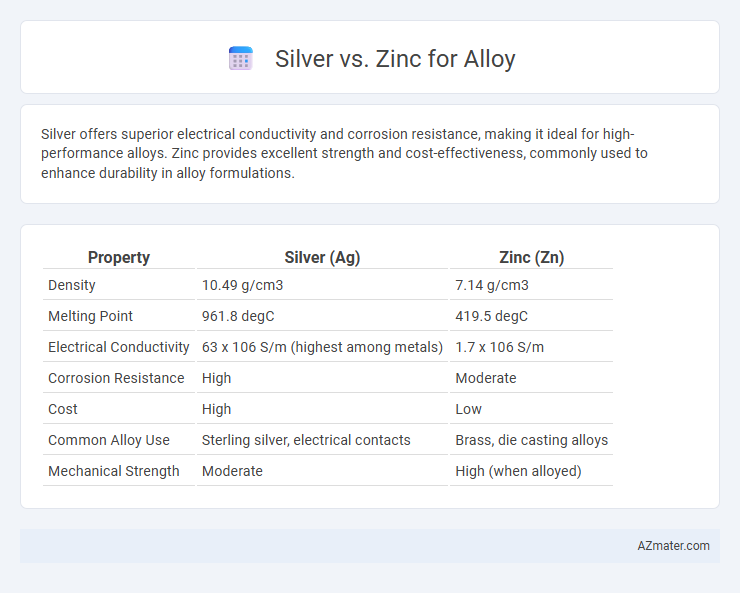
Silver offers superior electrical conductivity and corrosion resistance, making it ideal for high-performance alloys. Zinc provides excellent strength and cost-effectiveness, commonly used to enhance durability in alloy formulations.
Table of Comparison
| Property | Silver (Ag) | Zinc (Zn) |
|---|---|---|
| Density | 10.49 g/cm3 | 7.14 g/cm3 |
| Melting Point | 961.8 degC | 419.5 degC |
| Electrical Conductivity | 63 x 106 S/m (highest among metals) | 1.7 x 106 S/m |
| Corrosion Resistance | High | Moderate |
| Cost | High | Low |
| Common Alloy Use | Sterling silver, electrical contacts | Brass, die casting alloys |
| Mechanical Strength | Moderate | High (when alloyed) |
Introduction to Silver and Zinc Alloys
Silver alloys are prized for their excellent electrical conductivity, corrosion resistance, and aesthetic appeal, making them ideal for jewelry, electronics, and coinage. Zinc alloys offer superior strength, wear resistance, and low cost, commonly used in die-casting, automotive parts, and hardware. Combining silver and zinc in alloy form balances conductivity and mechanical durability, tailoring material properties for specialized industrial applications.
Chemical Properties of Silver vs. Zinc
Silver exhibits excellent chemical stability with low reactivity, resisting oxidation and corrosion due to its noble metal characteristics. Zinc, by contrast, is more chemically active, readily forming a protective oxide layer that enhances corrosion resistance but also contributes to galvanic interactions in alloys. The differing chemical reactivities influence the alloy's performance, with silver providing durability and conductivity, while zinc offers sacrificial protection and improved castability.
Mechanical Strength Comparison
Silver alloys typically exhibit lower mechanical strength compared to zinc alloys due to the softer and more ductile nature of silver. Zinc, often used as a primary component in die-casting alloys, provides higher tensile and yield strength, making it ideal for structural applications demanding durability. The crystalline structure and hardness of zinc-based alloys result in superior resistance to deformation and wear compared to silver-containing alloys.
Corrosion Resistance: Silver vs. Zinc
Silver offers superior corrosion resistance compared to zinc, maintaining its luster and structural integrity in various environments including exposure to air, moisture, and mild acids. Zinc, while providing decent protection through its sacrificial oxidation properties, tends to corrode more rapidly especially under harsh conditions like acidic or saline environments. Alloys containing silver typically exhibit enhanced durability and resistance to tarnishing, making them preferable for applications requiring long-term corrosion stability.
Electrical and Thermal Conductivity
Silver exhibits superior electrical conductivity at approximately 63 x 10^6 S/m, significantly outperforming zinc, which has around 17 x 10^6 S/m. Thermal conductivity also favors silver with values near 429 W/mK, compared to zinc's approximately 116 W/mK, making silver alloys ideal for applications requiring efficient heat dissipation. Zinc alloys, while less conductive, offer benefits in corrosion resistance and strength, but for maximizing conductivity, silver remains the preferred choice in electrical and thermal applications.
Common Applications of Silver Alloys
Silver alloys are widely used in jewelry, electronics, and dental applications due to their excellent conductivity, corrosion resistance, and antimicrobial properties. Common silver alloy applications include sterling silver for fine jewelry, silver-copper alloys in electrical contacts, and silver-palladium alloys in dental restorations. These applications leverage silver's superior luster, thermal conductivity, and biocompatibility compared to zinc-based alloys.
Common Applications of Zinc Alloys
Zinc alloys are widely used in die-casting for automotive parts, electrical components, and hardware due to their excellent strength, corrosion resistance, and cost-effectiveness compared to silver. Common applications include automotive gears, household fixtures, and electronic connectors, benefiting from zinc's ability to form complex shapes with tight tolerances. In contrast, silver is reserved for specialized uses requiring high electrical conductivity and aesthetic appeal, making zinc alloys the practical choice for mass-produced industrial components.
Cost and Availability of Silver and Zinc
Zinc is significantly more cost-effective than silver due to its abundant availability and lower extraction expenses, making it a preferred choice for large-scale alloy production. Silver, being a precious metal with limited reserves and higher market demand, commands a premium price, increasing the overall cost of alloys that contain it. The availability of zinc in numerous mining regions worldwide ensures consistent supply, while silver's scarcity and geopolitical factors can lead to fluctuating prices and supply challenges.
Environmental Impact and Sustainability
Silver-zinc alloys demonstrate a lower environmental impact compared to traditional silver alloys due to zinc's abundant availability and lower energy requirements for extraction and processing. Zinc's recyclability and reduced toxicity contribute to enhanced sustainability in alloy production, making silver-zinc alloys favorable for eco-friendly applications. The combination results in durable materials with a smaller carbon footprint, promoting responsible manufacturing and end-of-life recycling efforts.
Choosing the Right Alloy: Silver or Zinc
Choosing the right alloy between silver and zinc depends on the desired mechanical properties and application requirements. Silver alloys offer excellent conductivity, corrosion resistance, and aesthetic appeal, making them ideal for jewelry and electrical components. Zinc alloys provide superior strength, cost-effectiveness, and good casting properties, suitable for automotive, construction, and die-casting industries.
Infographic: Silver vs Zinc for Alloy
 azmater.com
azmater.com