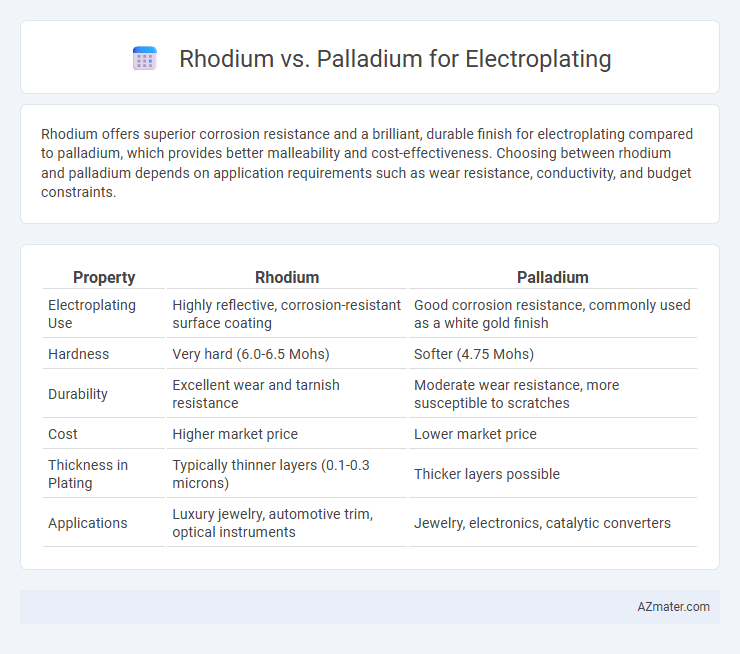
Rhodium offers superior corrosion resistance and a brilliant, durable finish for electroplating compared to palladium, which provides better malleability and cost-effectiveness. Choosing between rhodium and palladium depends on application requirements such as wear resistance, conductivity, and budget constraints.
Table of Comparison
| Property | Rhodium | Palladium |
|---|---|---|
| Electroplating Use | Highly reflective, corrosion-resistant surface coating | Good corrosion resistance, commonly used as a white gold finish |
| Hardness | Very hard (6.0-6.5 Mohs) | Softer (4.75 Mohs) |
| Durability | Excellent wear and tarnish resistance | Moderate wear resistance, more susceptible to scratches |
| Cost | Higher market price | Lower market price |
| Thickness in Plating | Typically thinner layers (0.1-0.3 microns) | Thicker layers possible |
| Applications | Luxury jewelry, automotive trim, optical instruments | Jewelry, electronics, catalytic converters |
Introduction to Rhodium and Palladium Electroplating
Rhodium and palladium electroplating are widely used in electronics, jewelry, and automotive industries due to their exceptional corrosion resistance and conductivity. Rhodium plating provides a brilliant, mirror-like finish and superior hardness, making it ideal for jewelry and decorative applications. Palladium electroplating offers excellent tarnish resistance and compatibility with gold alloys, often used in electronic components and catalytic converters.
Chemical Properties: Rhodium vs Palladium
Rhodium exhibits exceptional corrosion resistance and a high melting point of 1964degC, making it ideal for durable, reflective electroplating finishes. Palladium, with a melting point of 1555degC and excellent catalytic properties, offers superior malleability and corrosion resistance for applications requiring flexibility and conductivity. Both metals resist oxidation well, but rhodium's chemical inertness provides a more robust protection layer, while palladium's affinity for hydrogen absorption enhances specific industrial electroplating uses.
Electroplating Process Differences
Rhodium electroplating requires a specialized acidic electrolyte with a lower metal ion concentration compared to palladium, resulting in a thinner, more reflective coating ideal for jewelry and high-corrosion resistance applications. Palladium electroplating uses a mildly alkaline or acidic bath with a higher metal ion concentration, producing a thicker, more durable layer suited for electrical contacts and automotive parts. The plating current density and temperature settings also differ, with rhodium necessitating higher current densities and lower temperatures to achieve optimal brightness and hardness, while palladium operates efficiently at moderate current densities and temperatures for enhanced wear resistance.
Durability and Wear Resistance
Rhodium exhibits superior durability and wear resistance compared to palladium, making it an excellent choice for high-traffic jewelry and automotive parts. Its hardness and corrosion resistance contribute to a longer-lasting, tarnish-free finish, especially in harsh environments. Palladium offers good wear resistance but tends to wear down faster under heavy use, requiring more frequent re-plating for maintained appearance.
Color and Finish Comparisons
Rhodium electroplating delivers a bright, white, and highly reflective finish with excellent corrosion resistance, making it ideal for jewelry and decorative applications requiring a mirror-like appearance. Palladium, while also offering a silvery-white finish, presents a slightly warmer tone and is less reflective than rhodium but provides superior hardness and durability for functional plating. Both metals enhance the surface quality but rhodium's brilliance and superior tarnish resistance make it the preferred choice for achieving a pristine, long-lasting shine.
Cost Analysis: Rhodium vs Palladium
Rhodium electroplating commands a significantly higher cost compared to palladium due to its rarity and superior corrosion resistance, making it ideal for high-end jewelry and automotive catalysts. Palladium offers a more economical alternative with good tarnish resistance and moderate conductivity, often used in electronics and decorative applications. Market volatility impacts both metals' prices, but rhodium's price per gram can be several times higher than palladium, affecting overall plating project budgets.
Industrial and Jewelry Applications
Rhodium offers superior hardness and corrosion resistance compared to palladium, making it ideal for industrial electroplating where durability is critical. In jewelry applications, rhodium plating enhances the brightness and wear resistance of white gold and silver, while palladium provides a softer, more affordable alternative with good tarnish resistance. The choice between rhodium and palladium electroplating depends on the required hardness, cost considerations, and the specific aesthetic or functional properties desired in the finished product.
Environmental and Safety Considerations
Rhodium electroplating offers superior corrosion resistance and durability but involves toxic chemicals like rhodium chloride and requires stringent waste management to prevent environmental contamination. Palladium plating, while less toxic and more environmentally friendly, still necessitates careful handling of palladium salts and cyanide-based solutions used in some processes to minimize health risks. Both metals demand proper ventilation, protective equipment, and recycling protocols to reduce hazardous waste and occupational exposure in electroplating applications.
Maintenance and Longevity of Plated Surfaces
Rhodium electroplating offers superior maintenance benefits due to its exceptional corrosion resistance and hardness, significantly extending the longevity of plated surfaces compared to palladium. Palladium plating, while providing good tarnish resistance and a smoother finish, requires more frequent touch-ups and protective coatings to maintain its appearance over time. The durable, wear-resistant nature of rhodium enables longer-lasting protection with minimal maintenance, making it ideal for high-contact applications.
Choosing the Right Metal for Your Electroplating Needs
Rhodium offers superior corrosion resistance and a brilliant, reflective finish, making it ideal for high-end jewelry and automotive applications requiring durability and aesthetic appeal. Palladium provides excellent oxidation resistance with a slightly warmer tone, often preferred for electronics and catalysts where conductivity and cost-effectiveness are crucial. Selecting between rhodium and palladium depends on factors like desired appearance, corrosion resistance, conductivity, and budget constraints specific to the electroplating project.
Infographic: Rhodium vs Palladium for Electroplating
 azmater.com
azmater.com