Palladium offers superior catalytic efficiency and higher resistance to thermal degradation compared to aluminum when used in catalytic converters. Its exceptional ability to catalyze oxidation and reduction reactions makes palladium the preferred choice for reducing harmful emissions in automotive exhaust systems.
Table of Comparison
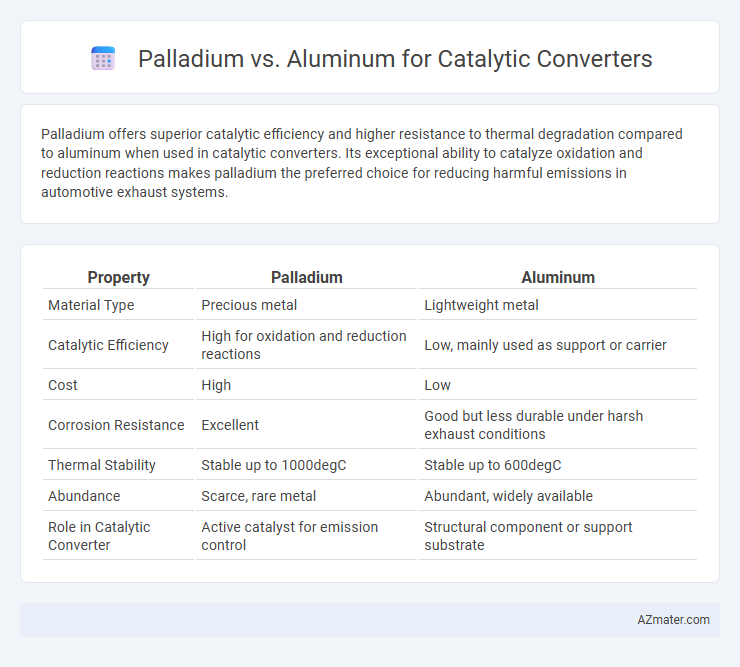
| Property | Palladium | Aluminum |
|---|---|---|
| Material Type | Precious metal | Lightweight metal |
| Catalytic Efficiency | High for oxidation and reduction reactions | Low, mainly used as support or carrier |
| Cost | High | Low |
| Corrosion Resistance | Excellent | Good but less durable under harsh exhaust conditions |
| Thermal Stability | Stable up to 1000degC | Stable up to 600degC |
| Abundance | Scarce, rare metal | Abundant, widely available |
| Role in Catalytic Converter | Active catalyst for emission control | Structural component or support substrate |
Introduction to Catalytic Converters
Catalytic converters use precious metals like palladium to efficiently convert harmful exhaust gases into less toxic emissions through oxidation and reduction reactions. Aluminum serves as the base material for the converter's substrate, providing a durable and lightweight honeycomb structure that supports the catalytic coating. The combination of a palladium catalyst on an aluminum substrate enhances the converter's durability and emission control efficiency in automotive applications.
Importance of Material Selection in Catalytic Converters
Palladium and aluminum play crucial roles in catalytic converters, with palladium serving as an active catalyst metal that efficiently reduces harmful emissions like nitrogen oxides, carbon monoxide, and hydrocarbons. Aluminum, primarily used in the converter's substrate or casing, provides structural stability, heat resistance, and corrosion protection essential for maintaining catalyst performance under extreme exhaust temperatures. Selecting the appropriate material combination enhances converter durability, emission control efficiency, and overall vehicle environmental compliance.
Overview of Palladium as a Catalyst
Palladium serves as a highly efficient catalyst in catalytic converters due to its exceptional ability to facilitate oxidation and reduction reactions at lower temperatures compared to aluminum. Its strong catalytic properties enable more effective conversion of harmful gases like carbon monoxide and hydrocarbons into less toxic emissions. Palladium's resistance to deactivation and sintering also contributes to its widespread use in automotive emission control technologies.
Overview of Aluminum in Catalytic Converter Applications
Aluminum plays a crucial role in catalytic converter applications primarily as a component of the ceramic or metallic substrate rather than as an active catalyst. Its high thermal stability, lightweight properties, and excellent corrosion resistance contribute to the structural integrity and durability of catalytic converters under extreme exhaust temperatures. Aluminum-based substrates provide a large surface area for catalyst deposition, enhancing the efficiency of palladium and other precious metals in reducing harmful vehicle emissions.
Comparative Catalytic Efficiency: Palladium vs Aluminum
Palladium exhibits significantly higher catalytic efficiency than aluminum in catalytic converters due to its superior ability to activate oxygen and hydrocarbons, promoting faster oxidation and reduction reactions. Unlike aluminum, which is primarily used as a structural component rather than a catalyst, palladium directly facilitates the critical conversion of harmful exhaust gases like CO, NOx, and hydrocarbons into less toxic substances. This catalytic superiority results in lower emissions and improved environmental compliance standards for vehicles utilizing palladium-based catalytic converters.
Durability and Longevity: Which Material Lasts Longer?
Palladium offers superior durability and longevity in catalytic converters due to its high resistance to oxidation and thermal degradation, maintaining catalytic activity over prolonged periods. Aluminum, primarily used in the substrate or housing rather than the catalyst itself, is less critical to longevity but contributes to the structural integrity through lightweight and corrosion resistance. Overall, catalytic converters with palladium catalysts demonstrate longer operational lifespan and sustained efficiency compared to those relying on less stable materials.
Cost Analysis: Palladium Versus Aluminum
Palladium is significantly more expensive than aluminum, with prices often reaching over $2,000 per ounce compared to aluminum's cost of around $2,000 per metric ton. Despite higher costs, palladium's superior catalytic properties justify its use in catalytic converters, while aluminum remains primarily a structural material due to its lower catalytic efficiency. Cost analysis must consider palladium's market volatility and supply constraints, impacting the overall expense of catalytic converter production in the automotive industry.
Environmental Impact of Palladium and Aluminum
Palladium, widely used in catalytic converters, offers exceptional catalytic efficiency but raises environmental concerns due to its rare extraction processes, which can lead to habitat destruction and high energy consumption. Aluminum, although less effective as a catalyst, has a significantly lower environmental footprint because of its abundance and recyclability, reducing the need for energy-intensive mining compared to palladium. Choosing aluminum-based components can mitigate environmental damage, but palladium remains critical for emissions control despite its ecological costs.
Market Trends and Availability
Palladium has become the preferred catalyst metal in catalytic converters due to tighter emissions regulations and its superior performance in converting harmful gases, driving a surge in demand and sustained high market prices. Aluminum, primarily used in the substrate rather than as a catalyst, remains abundant and cost-effective but does not directly influence emissions control efficiency like palladium. The increasing scarcity and geopolitical concerns around palladium supply have led to ongoing research into alternative materials and recycling strategies to mitigate market volatility.
Conclusion: Choosing the Right Material for Catalytic Converters
Palladium outperforms aluminum in catalytic converters due to its superior catalytic activity and higher resistance to poisoning, resulting in more efficient emission control. Aluminum, while lightweight and cost-effective, lacks the necessary chemical properties to catalyze harmful exhaust gases effectively. Selecting palladium ensures optimal durability and regulatory compliance for automotive emission standards.
Infographic: Palladium vs Aluminum for Catalytic Converter
 azmater.com
azmater.com