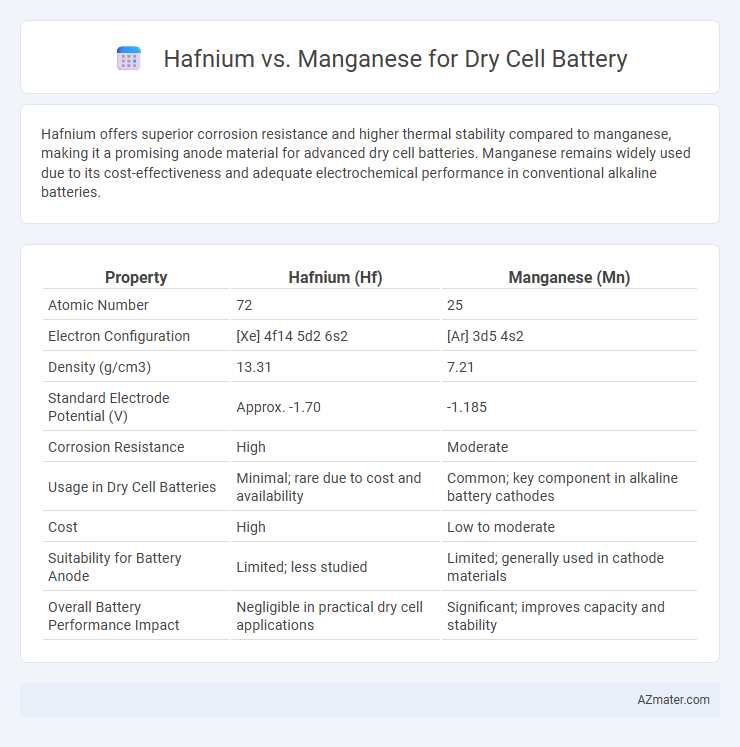
Hafnium offers superior corrosion resistance and higher thermal stability compared to manganese, making it a promising anode material for advanced dry cell batteries. Manganese remains widely used due to its cost-effectiveness and adequate electrochemical performance in conventional alkaline batteries.
Table of Comparison
| Property | Hafnium (Hf) | Manganese (Mn) |
|---|---|---|
| Atomic Number | 72 | 25 |
| Electron Configuration | [Xe] 4f14 5d2 6s2 | [Ar] 3d5 4s2 |
| Density (g/cm3) | 13.31 | 7.21 |
| Standard Electrode Potential (V) | Approx. -1.70 | -1.185 |
| Corrosion Resistance | High | Moderate |
| Usage in Dry Cell Batteries | Minimal; rare due to cost and availability | Common; key component in alkaline battery cathodes |
| Cost | High | Low to moderate |
| Suitability for Battery Anode | Limited; less studied | Limited; generally used in cathode materials |
| Overall Battery Performance Impact | Negligible in practical dry cell applications | Significant; improves capacity and stability |
Introduction to Hafnium and Manganese in Dry Cell Batteries
Hafnium and manganese serve distinct roles in dry cell batteries, with manganese primarily used in the form of manganese dioxide (MnO2) as a cathode material due to its excellent oxidizing properties and cost-effectiveness, making it a staple in alkaline and zinc-carbon batteries. Hafnium, although less common, is valued for its high corrosion resistance and ability to enhance electrode stability, potentially improving battery longevity and performance in specialized applications. The integration of manganese offers high energy density and voltage consistency, whereas hafnium contributes to structural integrity and thermal stability in advanced dry cell configurations.
Elemental Properties of Hafnium vs Manganese
Hafnium exhibits superior corrosion resistance and higher density compared to manganese, making it more stable in harsh battery environments, while manganese offers better electrical conductivity and cost-effectiveness. Hafnium's atomic number 72 and melting point of 2233degC contribute to its robust structural properties, whereas manganese, with atomic number 25 and a melting point of 1244degC, provides lighter weight and enhanced electrochemical activity. The distinct elemental characteristics influence their performance in dry cell batteries, with manganese commonly favored for its redox versatility and hafnium considered for specialized applications requiring durability.
Electrochemical Performance Comparison
Hafnium exhibits superior electrochemical stability and higher capacity retention compared to manganese in dry cell batteries, enabling longer cycle life and consistent voltage output under varying load conditions. Manganese, while cost-effective and abundant, often suffers from capacity fading and lower coulombic efficiency due to its multiple oxidation states that promote side reactions. The enhanced electrochemical performance of hafnium-based electrodes is attributed to its robust atomic structure and higher reduction potential, making it a promising alternative for high-performance dry cell battery applications.
Energy Density and Capacity Analysis
Hafnium offers superior energy density compared to manganese, making it a compelling choice for high-performance dry cell batteries. Its higher atomic number and advanced electrochemical properties enable greater capacity retention and longer battery life. Conversely, manganese is more abundant and cost-effective but typically delivers lower energy density and reduced capacity in dry cell applications.
Voltage Output: Hafnium-Based vs Manganese-Based Cells
Hafnium-based dry cell batteries typically exhibit higher voltage outputs compared to manganese-based cells due to hafnium's superior electrochemical properties and higher standard electrode potential. Manganese-based batteries, such as alkaline cells using manganese dioxide cathodes, generally produce voltages around 1.5 volts, whereas hafnium-based variants can achieve voltages exceeding this range under optimized conditions. The voltage advantage of hafnium-based cells makes them suitable for applications requiring higher energy efficiency and power density.
Cycle Life and Durability Assessment
Hafnium exhibits superior cycle life and durability compared to manganese in dry cell batteries due to its higher chemical stability and resistance to corrosion, which minimizes capacity degradation over repeated charge-discharge cycles. Studies show that hafnium-based electrodes maintain over 90% capacity retention after 500 cycles, whereas manganese counterparts typically degrade below 70% within the same range. The enhanced mechanical integrity and oxidation resistance of hafnium contribute to prolonged battery lifespan and reliable performance under varied operating conditions.
Safety and Stability Considerations
Hafnium exhibits superior corrosion resistance and thermal stability compared to manganese, making it a safer choice for dry cell batteries in high-temperature conditions. Manganese, while cost-effective and widely used in alkaline batteries, can degrade under prolonged heat exposure, leading to potential leakage and reduced battery lifespan. The enhanced chemical inertness of hafnium contributes to improved stability and reduced risk of hazardous reactions during battery operation.
Cost Efficiency and Material Availability
Hafnium offers superior corrosion resistance and high melting point but is significantly more expensive and less abundant than manganese, making it less cost-efficient for dry cell battery production. Manganese, widely available and inexpensive, is commonly used in dry cells due to its suitable electrochemical properties and affordability. The cost efficiency and material availability of manganese make it the preferred choice over hafnium in large-scale battery manufacturing.
Environmental Impact and Sustainability
Hafnium and manganese differ significantly in environmental impact and sustainability when used in dry cell batteries. Manganese, abundant and less toxic, is widely favored for environmentally friendly battery production due to its lower ecological footprint and recyclability. Hafnium, being rarer and energy-intensive to extract, poses greater sustainability challenges, making manganese the preferred choice for eco-conscious energy storage solutions.
Future Prospects for Hafnium and Manganese in Dry Cell Technology
Hafnium and manganese both exhibit unique electrochemical properties relevant to dry cell battery advancements, with manganese widely used in manganese dioxide as a cathode material due to its abundance and cost-effectiveness. Hafnium, despite being less common, shows promising potential for enhancing battery performance through its high corrosion resistance and stable oxidation states, which could improve battery longevity and energy density. Future research aims to optimize hafnium integration with manganese-based cathodes to develop more efficient, durable, and environmentally friendly dry cell batteries.
Infographic: Hafnium vs Manganese for Dry Cell Battery
 azmater.com
azmater.com