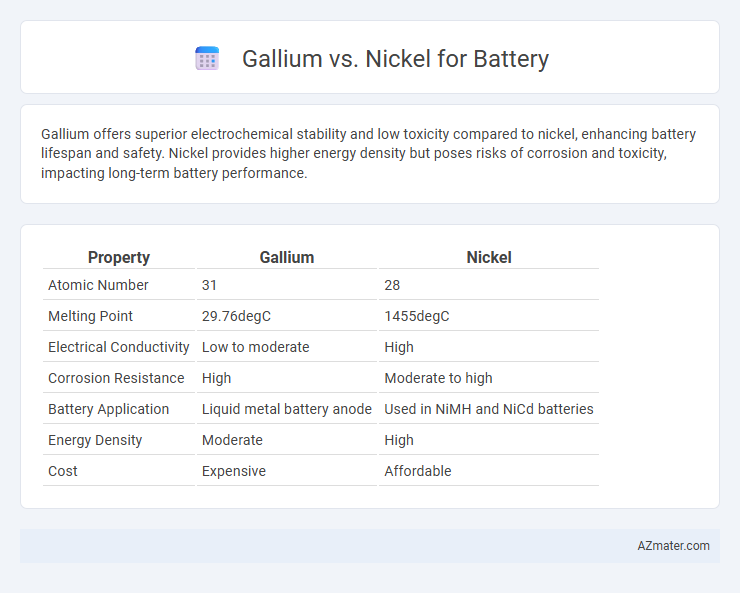
Gallium offers superior electrochemical stability and low toxicity compared to nickel, enhancing battery lifespan and safety. Nickel provides higher energy density but poses risks of corrosion and toxicity, impacting long-term battery performance.
Table of Comparison
| Property | Gallium | Nickel |
|---|---|---|
| Atomic Number | 31 | 28 |
| Melting Point | 29.76degC | 1455degC |
| Electrical Conductivity | Low to moderate | High |
| Corrosion Resistance | High | Moderate to high |
| Battery Application | Liquid metal battery anode | Used in NiMH and NiCd batteries |
| Energy Density | Moderate | High |
| Cost | Expensive | Affordable |
Introduction to Gallium and Nickel in Battery Technology
Gallium and nickel play distinct roles in battery technology, with gallium emerging as a promising material for enhancing battery efficiency and thermal stability. Nickel is widely used in battery cathodes, particularly in nickel-metal hydride (NiMH) and nickel-cobalt-aluminum (NCA) chemistries, due to its high energy density and cost-effectiveness. Gallium's ability to improve electrode performance and reduce dendrite formation positions it as a key element in the development of next-generation lithium-ion and solid-state batteries.
Chemical Properties and Conductivity
Gallium exhibits a low melting point of around 29.8degC and forms a stable oxide layer that enhances corrosion resistance, making it highly suitable for various battery applications. Nickel, with a melting point of 1455degC, offers robust chemical stability and excellent resistance to oxidation in alkaline environments commonly found in battery electrolytes. Conductivity-wise, nickel has a higher electrical conductivity (14.3 MS/m) compared to gallium (6.9 MS/m), influencing their effectiveness as electrode materials in battery systems.
Energy Density Comparison
Gallium-based batteries offer a promising energy density advantage over nickel-based batteries, primarily due to gallium's higher theoretical capacity and lower atomic weight. Nickel batteries, such as nickel-metal hydride (NiMH), typically achieve energy densities around 60-120 Wh/kg, whereas emerging gallium battery technologies aim to surpass 150 Wh/kg. This energy density improvement can lead to longer-lasting performance and lighter battery packs in applications requiring high power-to-weight ratios.
Cost and Availability of Materials
Gallium is significantly more expensive and less abundant than nickel, which makes nickel a more cost-effective choice for battery manufacturing. Nickel's widespread availability and established mining infrastructure contribute to its lower material costs, supporting large-scale battery production. Despite gallium's promising electrochemical properties, its limited supply and higher extraction costs hinder its commercial viability compared to nickel.
Environmental Impact and Sustainability
Gallium batteries offer enhanced recyclability and lower environmental toxicity compared to nickel-based batteries, reducing heavy metal pollution and hazardous waste challenges. Nickel mining is associated with significant ecological degradation, including deforestation, soil erosion, and water contamination, raising concerns about long-term sustainability. Gallium's abundance and less harmful extraction process contribute to a smaller carbon footprint, making it a more environmentally sustainable choice for battery technology development.
Performance in Rechargeable Batteries
Gallium enhances battery performance by improving energy density and thermal stability in rechargeable batteries, making it a promising additive for next-generation energy storage. Nickel is widely used in battery cathodes, especially in nickel-metal hydride (NiMH) and lithium nickel manganese cobalt oxide (NMC) batteries, offering high capacity and stability during repeated charge-discharge cycles. Gallium's ability to prevent dendrite formation and increase cycle life complements nickel's role in delivering robust electrical conductivity and structural integrity.
Safety and Thermal Stability
Gallium exhibits superior thermal stability compared to nickel, maintaining structural integrity at elevated temperatures, which reduces the risk of thermal runaway in batteries. Unlike nickel, gallium's low melting point enables it to absorb and dissipate heat efficiently, enhancing overall battery safety during high charge cycles. Nickel batteries, while commonly used, have higher risks of overheating and thermal instability, making gallium-based batteries a safer alternative for temperature-sensitive applications.
Scalability for Mass Production
Gallium offers promising potential for scalability in battery production due to its abundant availability and relatively low melting point, which enables energy-efficient processing techniques. Nickel, already established in large-scale battery manufacturing, benefits from a well-developed supply chain and cost-effective extraction methods, supporting high-volume production. However, gallium's unique electrochemical properties present opportunities for next-generation batteries that could complement or surpass nickel-based systems as production technologies mature.
Recent Innovations and Research Trends
Recent innovations in gallium for battery technology emphasize its low melting point and high conductivity, enabling flexible and safer liquid metal batteries with enhanced energy density and cycle life. Nickel-based batteries continue to dominate with improvements in nickel-rich cathodes that boost capacity and thermal stability in lithium-ion cells. Research trends highlight gallium's potential in solid-state and liquid metal batteries as a promising alternative, while nickel advancements focus on reducing cobalt content and enhancing sustainability in battery manufacturing.
Future Prospects in Battery Development
Gallium's unique ability to expand and contract without structural damage offers promising potential in next-generation battery anodes, enhancing cycle life and safety compared to traditional nickel-based batteries. Nickel remains a dominant choice due to its high energy density and established manufacturing processes but faces challenges like thermal runaway and material scarcity. Future battery development may see gallium alloys integrated to improve flexibility and longevity, potentially revolutionizing energy storage with safer, more durable alternatives.
Infographic: Gallium vs Nickel for Battery
 azmater.com
azmater.com