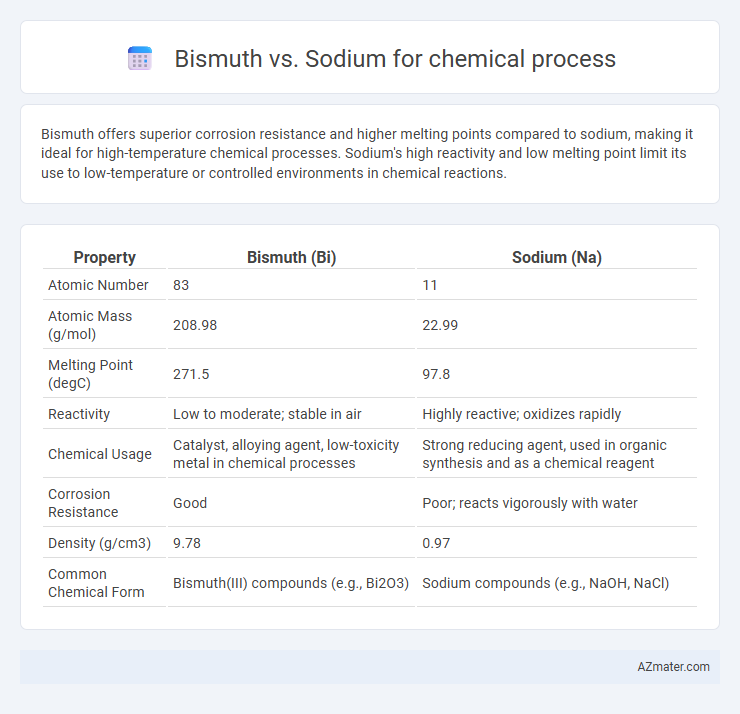
Bismuth offers superior corrosion resistance and higher melting points compared to sodium, making it ideal for high-temperature chemical processes. Sodium's high reactivity and low melting point limit its use to low-temperature or controlled environments in chemical reactions.
Table of Comparison
| Property | Bismuth (Bi) | Sodium (Na) |
|---|---|---|
| Atomic Number | 83 | 11 |
| Atomic Mass (g/mol) | 208.98 | 22.99 |
| Melting Point (degC) | 271.5 | 97.8 |
| Reactivity | Low to moderate; stable in air | Highly reactive; oxidizes rapidly |
| Chemical Usage | Catalyst, alloying agent, low-toxicity metal in chemical processes | Strong reducing agent, used in organic synthesis and as a chemical reagent |
| Corrosion Resistance | Good | Poor; reacts vigorously with water |
| Density (g/cm3) | 9.78 | 0.97 |
| Common Chemical Form | Bismuth(III) compounds (e.g., Bi2O3) | Sodium compounds (e.g., NaOH, NaCl) |
Overview of Bismuth and Sodium in Chemical Processes
Bismuth and sodium exhibit distinct roles in chemical processes due to their unique properties and reactivities. Bismuth is a heavy metal known for its low toxicity and high density, commonly used in pharmaceuticals, catalysts, and specialized alloys, often serving as a substitute for lead in environmentally safer applications. Sodium, an alkali metal characterized by high reactivity and strong reducing capabilities, is extensively employed in organic synthesis, metallurgy, and as a reagent in various reduction and deprotonation reactions, making it essential for industrial and laboratory chemical processes.
Physical and Chemical Properties Comparison
Bismuth exhibits a high density of 9.78 g/cm3 and a melting point of 271.5degC, contrasting with sodium's low density of 0.97 g/cm3 and melting point of 97.8degC, impacting their thermal stability in chemical processes. Chemically, bismuth is post-transition metal with poor electrical conductivity and high resistance to corrosion, making it suitable for applications requiring stability under oxidative conditions, while sodium is a highly reactive alkali metal with vigorous reactions in water and air, demanding stringent handling protocols. The difference in electronegativity, 2.02 for bismuth and 0.93 for sodium, influences their bonding behavior and reactivity, affecting their roles as reductants or catalysts in various reactions.
Reactivity and Stability in Industrial Applications
Bismuth exhibits lower reactivity compared to sodium, making it more stable under typical industrial chemical processes where controlled reactions are critical. Sodium's high reactivity with water and air demands stringent handling protocols and limits its use to processes requiring vigorous chemical activity, such as alkali production and organic synthesis. Industrial applications favor bismuth in environments where non-reactivity and material stability enhance safety and longevity in catalysts and alloys.
Environmental and Safety Considerations
Bismuth offers a significantly lower environmental impact and higher safety profile compared to sodium in chemical processes due to its non-toxic and non-reactive nature under ambient conditions. Sodium's high reactivity, especially with water, poses substantial fire and explosion hazards, requiring stringent handling protocols and specialized storage to prevent environmental contamination and human exposure. The use of bismuth minimizes hazardous waste and reduces the risk of accidental releases, making it a safer and more eco-friendly choice in industrial applications.
Performance as Catalysts in Chemical Reactions
Bismuth exhibits superior catalytic performance in selective oxidation and carbon-carbon coupling reactions due to its low toxicity and unique electronic properties, enabling high selectivity and stability under mild conditions. Sodium, often utilized as a strong reducing agent, enhances catalytic activity in hydrogenation and alkylation processes but suffers from high reactivity and poor selectivity, limiting its use in sensitive organic syntheses. The choice between bismuth and sodium catalysts largely depends on reaction specificity, with bismuth preferred for environmentally friendly oxidation reactions and sodium favored for aggressive reduction steps.
Cost and Availability in the Chemical Market
Bismuth is significantly more expensive than sodium due to its rarity and limited production, making it less economical for large-scale chemical processes. Sodium, abundant and widely available as a byproduct of salt, offers a cost-effective alternative with straightforward extraction methods supporting bulk industrial use. The chemical market favors sodium for applications where cost and availability directly impact process feasibility and scalability.
Handling, Storage, and Transportation Differences
Bismuth is a heavy, brittle metal requiring minimal reactive precautions, typically stored in airtight containers to prevent oxidation and handled with standard gloves due to low toxicity. Sodium is highly reactive, especially with water and air, demanding inert atmosphere storage like mineral oil or argon-filled containers and strict handling protocols with protective gear to avoid combustion or explosive reactions. Transportation of bismuth involves routine metal shipment methods, while sodium requires specialized packaging to isolate it from moisture and careful labeling to meet hazardous material regulations.
Applications in Organic and Inorganic Synthesis
Bismuth and sodium serve distinct roles in chemical processes, with bismuth often employed as a non-toxic catalyst in organic synthesis, facilitating reactions such as oxidation, reduction, and C-C bond formation due to its unique electronic properties. Sodium is widely used as a strong reducing agent in both organic and inorganic synthesis, enabling the preparation of sodium borohydride, sodium hydride, and various sodium-based reagents critical for reductions, deprotonations, and metalations. The choice between bismuth and sodium depends on reaction conditions, desired selectivity, and environmental considerations, with bismuth offering greener alternatives and sodium providing high reactivity under controlled environments.
Bismuth vs Sodium: Pros and Cons in Process Optimization
Bismuth offers high corrosion resistance and low toxicity, making it ideal for environmentally friendly chemical processes, though its higher cost and lower reactivity compared to sodium may limit large-scale applications. Sodium provides strong reducing properties and is cost-effective for energy-intensive reactions but poses safety risks due to its high reactivity and potential for hazardous byproducts. Process optimization must balance bismuth's stability and eco-friendliness against sodium's reactivity and affordability to achieve efficient and sustainable chemical manufacturing.
Future Trends and Innovations with Bismuth and Sodium
Bismuth and sodium are gaining significant attention in chemical processes due to their unique properties and environmental benefits. Research focuses on bismuth's non-toxicity and catalytic potential for green synthesis, while sodium's abundant availability and strong reducing power support advancements in energy storage and synthesis reactions. Emerging innovations emphasize bismuth-sodium hybrid systems to enhance efficiency and sustainability in battery technology and organic transformations.
Infographic: Bismuth vs Sodium for Chemical process
 azmater.com
azmater.com