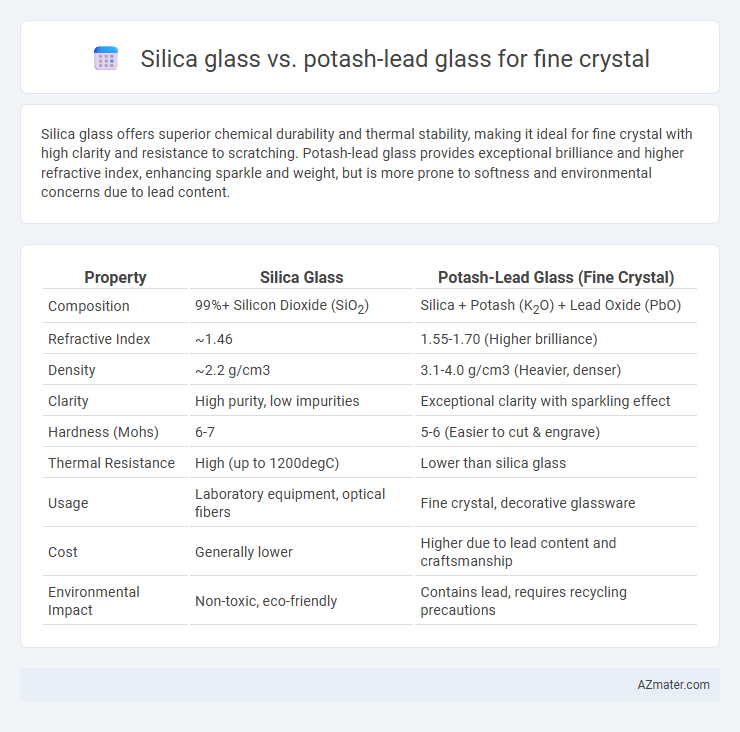
Silica glass offers superior chemical durability and thermal stability, making it ideal for fine crystal with high clarity and resistance to scratching. Potash-lead glass provides exceptional brilliance and higher refractive index, enhancing sparkle and weight, but is more prone to softness and environmental concerns due to lead content.
Table of Comparison
| Property | Silica Glass | Potash-Lead Glass (Fine Crystal) |
|---|---|---|
| Composition | 99%+ Silicon Dioxide (SiO2) | Silica + Potash (K2O) + Lead Oxide (PbO) |
| Refractive Index | ~1.46 | 1.55-1.70 (Higher brilliance) |
| Density | ~2.2 g/cm3 | 3.1-4.0 g/cm3 (Heavier, denser) |
| Clarity | High purity, low impurities | Exceptional clarity with sparkling effect |
| Hardness (Mohs) | 6-7 | 5-6 (Easier to cut & engrave) |
| Thermal Resistance | High (up to 1200degC) | Lower than silica glass |
| Usage | Laboratory equipment, optical fibers | Fine crystal, decorative glassware |
| Cost | Generally lower | Higher due to lead content and craftsmanship |
| Environmental Impact | Non-toxic, eco-friendly | Contains lead, requires recycling precautions |
Introduction to Fine Crystal Glass Types
Fine crystal glass primarily divides into silica glass and potash-lead glass, each offering distinct optical qualities and manufacturing processes. Silica glass, known for its high purity and durability, provides excellent clarity but tends to have lower refractive indices compared to potash-lead glass. Potash-lead glass, enriched with lead oxide, delivers superior brilliance and weight, making it the preferred choice for luxury fine crystal due to its enhanced light refraction and resonance properties.
What is Silica Glass?
Silica glass, also known as fused quartz, is a high-purity form of glass made primarily from silicon dioxide (SiO2), prized for its exceptional thermal stability, chemical inertness, and transparency to ultraviolet light. It differs from potash-lead glass, which contains lead oxide and potassium oxide, by offering superior resistance to thermal shock and a lower refractive index, resulting in a more muted sparkle compared to the pronounced brilliance of fine crystal lead glass. Silica glass is frequently used in applications requiring durability and optical clarity without lead's toxicity concerns, whereas potash-lead glass is favored for its high density and refractive properties in luxury glassware.
What is Potash-Lead Glass?
Potash-lead glass, commonly known as lead crystal, is a type of fine crystal glass containing substantial amounts of lead oxide, typically between 24% and 35%, which enhances its refractive index and brilliance. Compared to silica glass, potash-lead glass offers superior clarity, weight, and a characteristic sparkle that makes it ideal for luxury glassware and decorative items. The lead content increases the density and workability, allowing for intricate cuts and designs that are less achievable with traditional silica-based glass.
Chemical Composition Comparison
Silica glass primarily consists of silicon dioxide (SiO2), offering high chemical purity and thermal stability, whereas potash-lead glass contains significant amounts of lead oxide (PbO) and potassium oxide (K2O), which enhance refractive index and density. The addition of lead oxide in potash-lead glass increases brilliance and weight, making it a preferred choice for fine crystal applications due to its superior optical properties. Silica glass is favored for its durability and resistance to chemical corrosion, while potash-lead glass's enhanced dispersion contributes to its characteristic sparkle and clarity.
Optical Clarity and Brilliance
Silica glass offers exceptional optical clarity due to its high purity and low dispersion, resulting in minimal light distortion and a more transparent appearance. Potash-lead glass, enriched with lead oxide typically ranging from 24% to 30%, enhances brilliance through increased refractive index and higher dispersion, producing pronounced sparkle and rainbow-like light effects. Fine crystal made from potash-lead glass is renowned for its luminous brilliance, whereas silica glass excels in delivering pure clarity with less chromatic aberration.
Strength and Durability Factors
Silica glass, known for its high purity and hardness, offers superior scratch resistance and thermal stability, enhancing the strength and durability of fine crystal. Potash-lead glass, containing lead oxide and potash, provides greater density and brilliance but is generally more prone to scratching and less resistant to thermal shock compared to silica-based options. The structural composition of silica glass makes it ideal for applications demanding long-lasting clarity and mechanical toughness in fine crystal products.
Workability and Crafting Differences
Silica glass, known for its high melting point and chemical stability, offers exceptional workability through precise thermal shaping and grinding, making it ideal for intricate fine crystal designs. Potash-lead glass contains lead oxide, which lowers the melting temperature and increases refractive index, enhancing brilliance and ease of cutting with traditional tools, but demands careful handling due to its softness. Crafting with silica glass requires advanced kiln control for quality finishes, while potash-lead glass allows faster shaping and polishing processes, favored in artisanal crystal production.
Sustainability and Environmental Impact
Silica glass offers superior sustainability due to its abundant natural resources and lower toxicity compared to potash-lead glass, which contains heavy metals posing environmental hazards during production and disposal. The energy-intensive manufacturing process of potash-lead glass results in higher carbon emissions, while silica glass can be produced with more eco-friendly methods and is easier to recycle. Choosing silica glass for fine crystal reduces environmental impact by minimizing hazardous waste and supporting sustainable material use.
Applications in Fine Crystal Industry
Silica glass is prized in the fine crystal industry for its high thermal stability and excellent optical clarity, making it ideal for intricate glassware and decorative pieces requiring precision and durability. Potash-lead glass enhances brilliance and weight, offering superior refractive qualities that create the signature sparkle of luxury crystal items such as stemware, chandeliers, and ornamental figurines. The choice between silica and potash-lead glass depends on the desired balance of durability, optical performance, and aesthetic value in fine crystal production.
Conclusion: Choosing the Best Glass for Fine Crystal
Silica glass offers superior thermal stability and chemical durability, making it ideal for fine crystal with long-lasting clarity and resistance to etching. Potash-lead glass excels in brilliance and weight, providing exceptional refractive qualities that enhance the sparkle and elegance of fine crystal pieces. Selecting the best glass depends on prioritizing either durability and purity with silica glass or optical brilliance and traditional aesthetics with potash-lead glass.
Infographic: Silica glass vs Potash-lead glass for Fine crystal
 azmater.com
azmater.com