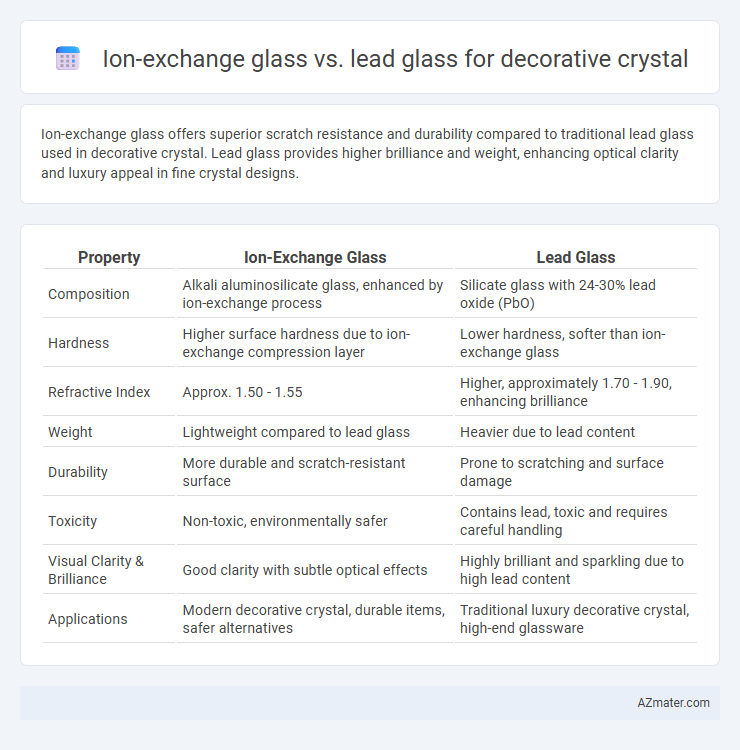
Ion-exchange glass offers superior scratch resistance and durability compared to traditional lead glass used in decorative crystal. Lead glass provides higher brilliance and weight, enhancing optical clarity and luxury appeal in fine crystal designs.
Table of Comparison
| Property | Ion-Exchange Glass | Lead Glass |
|---|---|---|
| Composition | Alkali aluminosilicate glass, enhanced by ion-exchange process | Silicate glass with 24-30% lead oxide (PbO) |
| Hardness | Higher surface hardness due to ion-exchange compression layer | Lower hardness, softer than ion-exchange glass |
| Refractive Index | Approx. 1.50 - 1.55 | Higher, approximately 1.70 - 1.90, enhancing brilliance |
| Weight | Lightweight compared to lead glass | Heavier due to lead content |
| Durability | More durable and scratch-resistant surface | Prone to scratching and surface damage |
| Toxicity | Non-toxic, environmentally safer | Contains lead, toxic and requires careful handling |
| Visual Clarity & Brilliance | Good clarity with subtle optical effects | Highly brilliant and sparkling due to high lead content |
| Applications | Modern decorative crystal, durable items, safer alternatives | Traditional luxury decorative crystal, high-end glassware |
Introduction to Decorative Crystal Materials
Ion-exchange glass and lead glass serve as prominent materials in the production of decorative crystal, each offering distinct optical properties and durability. Ion-exchange glass is known for its enhanced surface strength and scratch resistance, achieved through a chemical strengthening process that replaces smaller ions with larger ones in the glass matrix. Lead glass, containing high lead oxide content, is prized for its exceptional brilliance and high refractive index, producing a sparkling effect that makes it a preferred choice in luxury decorative crystal products.
Overview of Ion-Exchange Glass
Ion-exchange glass enhances decorative crystal by incorporating a chemical process that replaces smaller ions in the glass with larger ones, increasing surface compressive stress and significantly improving durability and scratch resistance. Unlike lead glass, which is prized for its high refractive index and brilliance but is softer and more prone to damage, ion-exchange glass offers a more robust alternative with superior strength and safety due to the absence of toxic lead. This technology allows decorative crystal to maintain clarity and sparkle while providing enhanced longevity and resilience in daily use.
Overview of Lead Glass
Lead glass, renowned for its high refractive index and exceptional brilliance, contains 24-30% lead oxide, which enhances its weight and clarity compared to ion-exchange glass. This type of glass is prized in decorative crystal applications for its superior light dispersion, creating vivid sparkle and a luxurious appearance. While ion-exchange glass offers improved surface hardness, lead glass remains the preferred choice for ornamental crystal pieces due to its unmatched optical properties and visual appeal.
Chemical Composition Differences
Ion-exchange glass for decorative crystal primarily consists of aluminosilicate compositions with sodium ions replaced by larger potassium ions to enhance surface strength and durability. Lead glass contains significant amounts of lead oxide (typically 18-30%), which increases its refractive index and density, giving it a brilliant sparkle and heaviness. The substitution of lead oxide in lead glass results in lower melting points and greater ease of cutting compared to the chemically strengthened ion-exchange glass.
Visual and Optical Properties
Ion-exchange glass exhibits superior scratch resistance and enhanced surface hardness while maintaining excellent optical clarity and high light transmission, contributing to its brilliance in decorative crystal applications. Lead glass, renowned for its high refractive index and exceptional dispersion, offers unmatched brilliance, sparkle, and a warm glow due to its lead oxide content. The choice between ion-exchange and lead glass depends on the desired balance between durability and optimal light refraction for decorative crystal pieces.
Durability and Strength Comparison
Ion-exchange glass exhibits superior durability and increased surface strength compared to lead glass due to its chemically strengthened surface layer formed through the ion-exchange process. This treatment enhances resistance to scratches, impacts, and everyday wear, making ion-exchange glass more suitable for long-lasting decorative crystal applications. Lead glass, while prized for its high refractive index and brilliance, is inherently softer and more prone to damage, reducing its overall durability in decorative contexts.
Environmental and Health Considerations
Ion-exchange glass for decorative crystal offers a safer alternative to lead glass by eliminating the risk of lead poisoning and environmental contamination associated with lead leaching. Ion-exchange processes enhance glass strength without relying on toxic heavy metals, making the material more eco-friendly and suitable for households concerned with sustainability. In contrast, lead glass, while prized for its brilliance and weight, poses significant health risks during manufacturing and disposal due to lead's toxicity and environmental persistence.
Design Flexibility and Artistic Applications
Ion-exchange glass offers superior design flexibility compared to lead glass due to its enhanced chemical durability and ability to withstand intricate shaping and etching processes without compromising structural integrity. This durability facilitates complex artistic applications such as detailed engravings, delicate filigree, and vibrant color variations that are challenging to achieve with lead glass. While lead glass provides exceptional brilliance and weight, ion-exchange glass expands creative possibilities for decorative crystal by enabling innovative textures and multidimensional designs.
Cost and Availability
Ion-exchange glass offers a cost-effective alternative to lead glass, with a lower manufacturing expense due to the absence of toxic lead components, making it more accessible for large-scale decorative crystal production. Lead glass, known for its superior brilliance and weight, tends to be more expensive and may face regulatory restrictions impacting availability in certain markets. The broader availability of ion-exchange glass, combined with competitive pricing, supports its growing use as a sustainable substitute for traditional lead crystal in the decorative industry.
Choosing the Right Glass for Decorative Crystal
Selecting ion-exchange glass for decorative crystal ensures enhanced durability and scratch resistance due to its chemically strengthened surface, making it ideal for high-traffic or frequently handled pieces. Lead glass offers superior clarity and a higher refractive index, providing brilliant sparkle and weight that classic crystal enthusiasts value. Understanding the balance between longevity and optical brilliance is crucial when choosing the right glass for decorative crystal applications.
Infographic: Ion-exchange glass vs Lead glass for Decorative crystal
 azmater.com
azmater.com