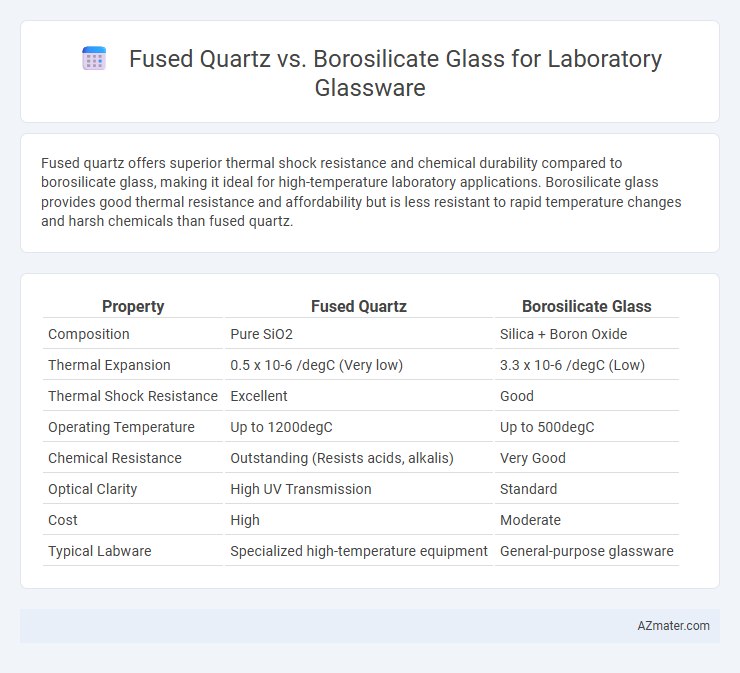
Fused quartz offers superior thermal shock resistance and chemical durability compared to borosilicate glass, making it ideal for high-temperature laboratory applications. Borosilicate glass provides good thermal resistance and affordability but is less resistant to rapid temperature changes and harsh chemicals than fused quartz.
Table of Comparison
| Property | Fused Quartz | Borosilicate Glass |
|---|---|---|
| Composition | Pure SiO2 | Silica + Boron Oxide |
| Thermal Expansion | 0.5 x 10-6 /degC (Very low) | 3.3 x 10-6 /degC (Low) |
| Thermal Shock Resistance | Excellent | Good |
| Operating Temperature | Up to 1200degC | Up to 500degC |
| Chemical Resistance | Outstanding (Resists acids, alkalis) | Very Good |
| Optical Clarity | High UV Transmission | Standard |
| Cost | High | Moderate |
| Typical Labware | Specialized high-temperature equipment | General-purpose glassware |
Introduction to Laboratory Glassware Materials
Fused quartz offers exceptional thermal resistance, chemical inertness, and high purity, making it ideal for laboratory glassware requiring extreme temperature stability and minimal contamination. Borosilicate glass, known for its excellent thermal shock resistance and affordability, is widely used in labs for general-purpose glassware such as beakers, flasks, and pipettes. Both materials balance durability and chemical resistance, but fused quartz is preferred for high-precision applications involving aggressive chemicals or high-temperature processes.
Overview of Fused Quartz
Fused quartz is a high-purity, non-crystalline form of silicon dioxide known for its exceptional thermal stability, low thermal expansion, and outstanding chemical resistance, making it ideal for laboratory glassware exposed to extreme temperatures and corrosive chemicals. It withstands temperatures up to 1200degC, surpassing borosilicate glass, which typically tolerates around 500degC, providing greater durability for high-heat applications. Its optical clarity and low thermal expansion coefficient (approximately 0.5 x 10-6 /degC) ensure minimal distortion during rapid temperature changes, enhancing its performance in precise scientific experiments.
Overview of Borosilicate Glass
Borosilicate glass is widely used in laboratory glassware due to its excellent thermal resistance and chemical durability, with a low coefficient of thermal expansion around 3.3 x 10^-6 /degC, which minimizes thermal shock risk. It is made from silica and boron trioxide, offering superior resistance to acids, alkalis, and organic solvents, making it suitable for various chemical applications. Compared to fused quartz, borosilicate glass is more cost-effective and easier to produce in complex shapes, although it has slightly lower thermal and mechanical strength.
Chemical Resistance: Fused Quartz vs Borosilicate
Fused quartz exhibits superior chemical resistance compared to borosilicate glass, resisting strong acids, alkalis, and organic solvents without degradation. Borosilicate glass, while chemically durable, can be etched or corroded by hydrofluoric acid and concentrated alkalis over time. The high purity and silica content in fused quartz make it the preferred choice in environments requiring extreme chemical inertness and contamination-free reactions.
Thermal Properties and Heat Tolerance
Fused quartz offers superior thermal properties with a high melting point around 1,710degC and exceptional thermal shock resistance, making it ideal for applications requiring rapid temperature changes. Borosilicate glass, with a lower melting point near 820degC, provides good thermal resistance but is less tolerant of extreme temperature fluctuations compared to fused quartz. The coefficient of thermal expansion for fused quartz is significantly lower (~0.5 x 10^-6 /degC) than borosilicate glass (~3.3 x 10^-6 /degC), enhancing its durability under high heat conditions in laboratory settings.
Mechanical Strength and Durability
Fused quartz exhibits superior mechanical strength with a high modulus of elasticity and exceptional resistance to thermal shock, making it ideal for rigorous laboratory applications requiring durability. Borosilicate glass offers good mechanical strength combined with excellent chemical resistance and thermal stability, though it is more prone to fracturing under extreme temperature changes compared to fused quartz. The enhanced durability of fused quartz under mechanical stress and rapid temperature fluctuations makes it preferable when maximum structural integrity is essential in laboratory glassware.
Optical Clarity and Light Transmission
Fused quartz offers superior optical clarity and transmits ultraviolet (UV) light more effectively than borosilicate glass, making it ideal for applications requiring high UV transparency and minimal light distortion. Borosilicate glass provides good optical clarity but has lower UV transmission and more absorption in the ultraviolet range compared to fused quartz. For precise optical measurements and UV-sensitive experiments, fused quartz laboratory glassware is preferred due to its exceptional light transmission properties and minimal fluorescence.
Cost Comparison and Budget Considerations
Fused quartz glassware generally incurs higher costs due to its superior thermal shock resistance and chemical durability, making it ideal for high-temperature or highly reactive laboratory applications. Borosilicate glass offers a more economical option with adequate thermal and chemical resistance for routine laboratory use, supporting budget-conscious purchases without compromising safety. Laboratories balancing performance requirements and budget constraints often prefer borosilicate glass for everyday tasks while reserving fused quartz for specialized experiments to optimize overall expenditure.
Common Applications in Laboratories
Fused quartz is highly valued in laboratory settings for applications requiring exceptional thermal shock resistance and high purity, such as spectroscopy, high-temperature furnaces, and UV optical components. Borosilicate glass is commonly used in general-purpose laboratory glassware like beakers, flasks, and condensers due to its excellent chemical resistance and moderate thermal durability. Both materials are chosen based on specific experimental needs, with fused quartz preferred for extreme temperature and optical clarity, while borosilicate is favored for cost-effective, versatile lab equipment.
Choosing the Right Material for Your Lab
Fused quartz offers superior thermal resistance, minimal thermal expansion, and excellent chemical durability, making it ideal for high-temperature applications in laboratories requiring precise measurements. Borosilicate glass provides excellent thermal shock resistance, good chemical stability, and cost-effectiveness, suitable for general-purpose labware exposed to rapid temperature changes. Selecting the right material depends on specific application needs such as temperature range, chemical exposure, and budget constraints to ensure durability and accuracy in laboratory experiments.
Infographic: Fused quartz vs Borosilicate glass for Laboratory glassware
 azmater.com
azmater.com