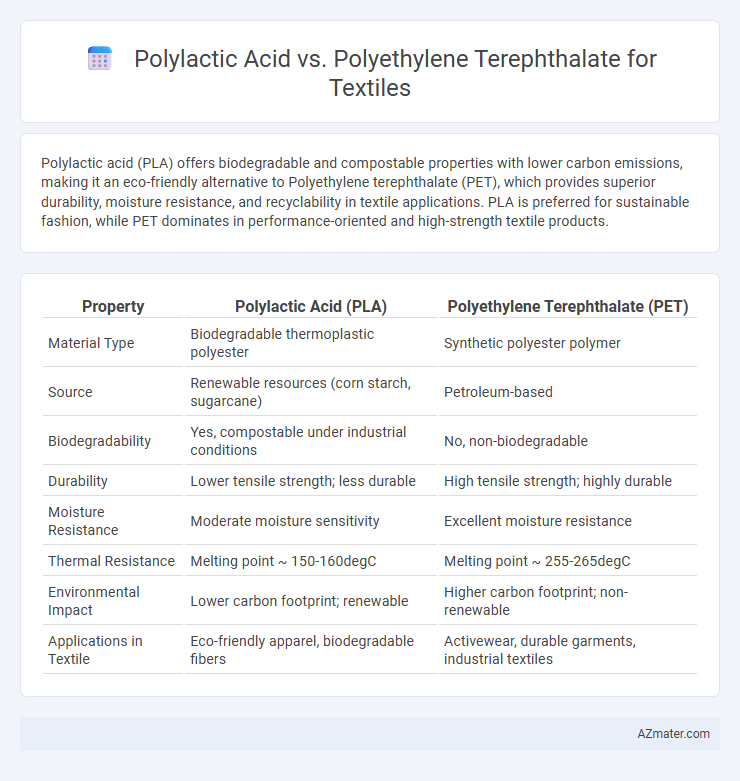
Polylactic acid (PLA) offers biodegradable and compostable properties with lower carbon emissions, making it an eco-friendly alternative to Polyethylene terephthalate (PET), which provides superior durability, moisture resistance, and recyclability in textile applications. PLA is preferred for sustainable fashion, while PET dominates in performance-oriented and high-strength textile products.
Table of Comparison
| Property | Polylactic Acid (PLA) | Polyethylene Terephthalate (PET) |
|---|---|---|
| Material Type | Biodegradable thermoplastic polyester | Synthetic polyester polymer |
| Source | Renewable resources (corn starch, sugarcane) | Petroleum-based |
| Biodegradability | Yes, compostable under industrial conditions | No, non-biodegradable |
| Durability | Lower tensile strength; less durable | High tensile strength; highly durable |
| Moisture Resistance | Moderate moisture sensitivity | Excellent moisture resistance |
| Thermal Resistance | Melting point ~ 150-160degC | Melting point ~ 255-265degC |
| Environmental Impact | Lower carbon footprint; renewable | Higher carbon footprint; non-renewable |
| Applications in Textile | Eco-friendly apparel, biodegradable fibers | Activewear, durable garments, industrial textiles |
Introduction to Polylactic Acid (PLA) and Polyethylene Terephthalate (PET) in Textiles
Polylactic acid (PLA) is a biodegradable, bio-based polymer derived from renewable resources like corn starch, widely used in textiles for its eco-friendly characteristics and breathability. Polyethylene terephthalate (PET), a petroleum-based synthetic polymer, dominates the textile industry due to its strength, durability, and moisture-wicking properties. While PLA offers sustainability advantages, PET remains favored for high-performance fabric applications, balancing environmental impact with functionality.
Chemical Structure and Properties: PLA vs PET
Polylactic acid (PLA) is a biodegradable polyester derived from renewable resources, characterized by its aliphatic linear polymer chain consisting of lactic acid units, which provides lower thermal stability and reduced moisture resistance compared to polyethylene terephthalate (PET). PET is a synthetic aromatic polyester composed of repeating ethylene terephthalate units, offering superior tensile strength, chemical resistance, and durability for textile applications due to its rigid benzene ring structure. The distinct chemical architectures of PLA and PET directly influence their mechanical properties, biodegradability, and suitability for various textile manufacturing processes.
Production Processes and Raw Materials
Polylactic acid (PLA) is produced through the fermentation of renewable biomass sources such as corn starch or sugarcane, involving polymerization of lactic acid derived from these organic feedstocks. In contrast, Polyethylene terephthalate (PET) is synthesized from petrochemical raw materials, primarily purified terephthalic acid (PTA) and ethylene glycol, through a polycondensation reaction. The PLA production process emphasizes biodegradability and sustainability due to its bio-based origins, while PET relies on non-renewable fossil fuels and energy-intensive refining methods.
Mechanical Performance in Textile Applications
Polylactic acid (PLA) offers high tensile strength and excellent biodegradability, making it suitable for eco-friendly textile applications, whereas polyethylene terephthalate (PET) provides superior abrasion resistance and wrinkle retention essential for durable fabric performance. PLA fibers exhibit lower elongation at break compared to PET, resulting in less flexibility but higher stiffness, which affects garment comfort and shape retention. PET's higher melting point enhances its thermal stability, lending itself better to high-temperature textile processing and performance under mechanical stress.
Biodegradability and Environmental Impact Comparison
Polylactic acid (PLA) offers superior biodegradability compared to Polyethylene terephthalate (PET), as PLA can decompose into natural elements within weeks to months under industrial composting conditions, whereas PET persists for hundreds of years in landfills and marine environments. PLA's plant-based origin reduces carbon footprint and reliance on fossil fuels, enhancing its environmental sustainability over petroleum-derived PET, which contributes to microplastic pollution and greenhouse gas emissions during production and degradation. While PET provides durability and recyclability, the environmental benefits of PLA in textile applications are significant due to its renewability and lower end-of-life ecological impact.
Textile Processing and Fabrication Differences
Polylactic acid (PLA) offers biodegradability and lower melting points around 150-170degC, facilitating energy-efficient melt spinning in textile processing, whereas Polyethylene terephthalate (PET) requires higher processing temperatures near 250degC for fiber extrusion, impacting energy consumption. PLA fibers exhibit lower moisture regain and biodegradation rates useful for eco-friendly textile applications, but PET fibers demonstrate superior mechanical strength, dimensional stability, and resistance to chemical and UV degradation crucial for durable fabric fabrication. Differences in thermal properties influence finishing treatments, with PET accommodating higher-temperature processes such as heat setting and dye fixation, while PLA demands gentler bio-based finishing agents to maintain fiber integrity.
Thermal and Chemical Resistance
Polylactic acid (PLA) exhibits lower thermal resistance with a melting point around 150-160degC, making it less suitable for high-heat textile applications compared to polyethylene terephthalate (PET), which has a melting point near 260degC. In terms of chemical resistance, PET demonstrates superior durability against solvents, acids, and alkalis, while PLA tends to degrade or hydrolyze under similar chemical exposures. This thermal and chemical resilience makes PET the preferred choice for performance textiles subjected to harsh environmental conditions.
Dyeability and Aesthetic Qualities
Polylactic acid (PLA) fibers exhibit superior dyeability compared to polyethylene terephthalate (PET) due to their amorphous structure, allowing vibrant and uniform color absorption that enhances fabric aesthetics. PLA's inherent biodegradability and smooth surface contribute to a matte finish with excellent drape, while PET fibers produce a glossy, resilient texture with less dye uptake, often requiring disperse dyes under high temperature. The choice between PLA and PET for textiles hinges on prioritizing eco-friendly, richly colored materials versus durable, high-sheen fabrics with established industrial dyeing processes.
Cost Analysis and Market Availability
Polylactic acid (PLA) generally incurs higher production costs than polyethylene terephthalate (PET), primarily due to its bio-based raw materials and less mature manufacturing infrastructure. PET dominates the textile market with extensive availability, benefiting from well-established supply chains and economies of scale. Cost efficiency and widespread market presence make PET the preferred choice for large-scale textile production, while PLA serves niche segments emphasizing sustainability despite its premium pricing.
Future Trends and Innovations in PLA and PET Textiles
Polylactic acid (PLA) and Polyethylene terephthalate (PET) are advancing in textile applications with innovations emphasizing sustainability and performance enhancement. PLA fibers are gaining traction through bioplastic composites and improvements in biodegradability, offering eco-friendly alternatives for fashion and technical textiles. PET continues to evolve with recycled content integration and enhanced durability, supporting circular economy models in textile manufacturing.
Infographic: Polylactic acid vs Polyethylene terephthalate for Textile
 azmater.com
azmater.com