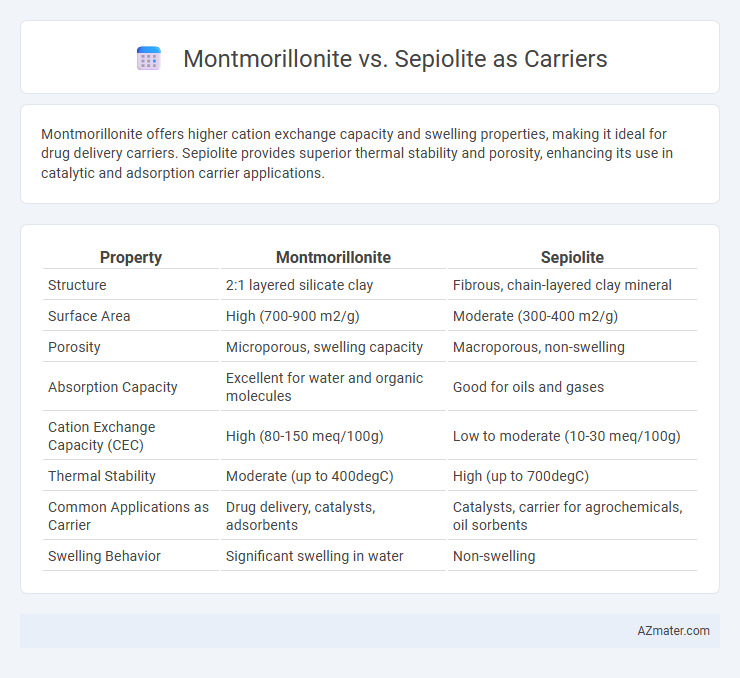
Montmorillonite offers higher cation exchange capacity and swelling properties, making it ideal for drug delivery carriers. Sepiolite provides superior thermal stability and porosity, enhancing its use in catalytic and adsorption carrier applications.
Table of Comparison
| Property | Montmorillonite | Sepiolite |
|---|---|---|
| Structure | 2:1 layered silicate clay | Fibrous, chain-layered clay mineral |
| Surface Area | High (700-900 m2/g) | Moderate (300-400 m2/g) |
| Porosity | Microporous, swelling capacity | Macroporous, non-swelling |
| Absorption Capacity | Excellent for water and organic molecules | Good for oils and gases |
| Cation Exchange Capacity (CEC) | High (80-150 meq/100g) | Low to moderate (10-30 meq/100g) |
| Thermal Stability | Moderate (up to 400degC) | High (up to 700degC) |
| Common Applications as Carrier | Drug delivery, catalysts, adsorbents | Catalysts, carrier for agrochemicals, oil sorbents |
| Swelling Behavior | Significant swelling in water | Non-swelling |
Introduction to Montmorillonite and Sepiolite as Carriers
Montmorillonite and Sepiolite are clay minerals widely utilized as carriers due to their high surface area and excellent adsorption properties. Montmorillonite features a layered structure with significant cation exchange capacity, making it ideal for hosting various molecules and enhancing stability. Sepiolite's fibrous morphology and large pore volume provide superior loading capacity and controlled release characteristics, advantageous for drug delivery and catalyst support applications.
Chemical Structure Comparison
Montmorillonite and Sepiolite are both layered silicate minerals with distinct chemical structures affecting their carrier properties; Montmorillonite features a 2:1 phyllosilicate structure with two tetrahedral silica sheets sandwiching an octahedral alumina sheet, allowing high cation exchange capacity and swelling behavior. Sepiolite has a fibrous, chain-like structure composed of alternating blocks of 2:1 layers and inversion tetrahedral sheets, resulting in a porous framework with high surface area but lower swelling compared to Montmorillonite. These structural differences influence their adsorption capacity, ion exchange potential, and suitability as carriers in applications like drug delivery and pollutant adsorption.
Physical Properties and Morphology
Montmorillonite exhibits a layered, expandable structure with a high cation exchange capacity and a surface area typically ranging from 200 to 800 m2/g, making it ideal for adsorptive carrier applications. Sepiolite possesses a fibrous, needle-like morphology with a high porosity and surface area around 300 m2/g, offering excellent mechanical strength and liquid absorption properties. The physical properties of Montmorillonite favor swelling and interlayer hosting, while Sepiolite's rigid fibrous framework provides enhanced structural integrity and flow-through channels for carrier systems.
Adsorption Capacities
Montmorillonite exhibits superior adsorption capacities compared to sepiolite due to its higher specific surface area and swelling properties, enabling enhanced uptake of organic and inorganic molecules. Sepiolite, with its fibrous morphology and stable structure, offers selective adsorption for larger molecules but generally lower capacity than montmorillonite. The distinct pore size distribution and cation exchange capacity in montmorillonite contribute significantly to its effectiveness as a carrier in adsorption applications.
Surface Area and Porosity
Montmorillonite exhibits a higher specific surface area, often exceeding 700 m2/g, which enhances its adsorption capacity and facilitates better carrier performance in catalytic and drug delivery systems. Sepiolite features a unique fibrous morphology with a well-developed porosity and mesoporous channels, typically offering a surface area around 200-300 m2/g, providing efficient molecular diffusion and storage. The choice between Montmorillonite and Sepiolite as carriers depends on the required balance between surface area and pore structure for targeted applications.
Compatibility with Active Ingredients
Montmorillonite exhibits a high cation exchange capacity, enabling strong adsorption and improved compatibility with a wide range of active ingredients, especially metal ions and organic molecules. Sepiolite, with its fibrous structure and large surface area, offers excellent adsorption but often shows selective affinity, favoring neutral or polar compounds over ionic species. Compatibility differences influence the choice of carrier depending on the physicochemical properties of active ingredients, impacting stability, release rate, and bioavailability in formulations.
Environmental Stability and Safety
Montmorillonite exhibits high environmental stability due to its layered structure and strong cation exchange capacity, which enhances its ability to retain nutrients and contaminants safely within its matrix. Sepiolite offers excellent safety profile as a carrier, characterized by low toxicity and high adsorption capacity, making it effective in immobilizing pollutants under diverse environmental conditions. Both clays provide robust carrier properties, but Montmorillonite's superior swelling capacity and chemical stability often make it more suitable for long-term environmental applications requiring sustained release and safety.
Cost and Availability
Montmorillonite, known for its high cation exchange capacity, is generally more abundant and cost-effective compared to Sepiolite, making it a preferred choice as a carrier in industrial applications. Sepiolite's fibrous structure offers unique adsorption properties but often comes at a higher cost due to limited global deposits and more complex extraction processes. The cost-efficiency and widespread availability of Montmorillonite give it an advantage in large-scale carrier applications where budget constraints are critical.
Application Fields and Effectiveness
Montmorillonite and Sepiolite are both highly effective clay minerals used as carriers in various application fields such as pharmaceuticals, agriculture, and environmental remediation. Montmorillonite offers superior adsorption capacity and swelling properties, making it ideal for drug delivery systems and contaminant removal, while Sepiolite's fibrous structure provides excellent viscosity and stabilization benefits, enhancing formulations in cosmetics and paints. Their distinct porosity and surface area characteristics determine their effectiveness, with Montmorillonite excelling in moisture retention and ion exchange, and Sepiolite optimizing mechanical strength and thermal stability.
Conclusion: Choosing the Optimal Carrier
Montmorillonite offers superior cation exchange capacity and swelling properties, making it highly effective for adsorbing and carrying charged molecules, whereas Sepiolite provides exceptional thermal stability and a unique fibrous structure ideal for high-temperature or mechanical stress environments. Selecting the optimal carrier depends on the specific application requirements, such as adsorption capacity, thermal behavior, and mechanical strength, with Montmorillonite favored for applications needing high surface area and ion exchange, while Sepiolite suits contexts demanding robustness and heat resistance. Balancing these properties ensures enhanced performance in catalysis, drug delivery, or environmental remediation systems.
Infographic: Montmorillonite vs Sepiolite for Carrier
 azmater.com
azmater.com