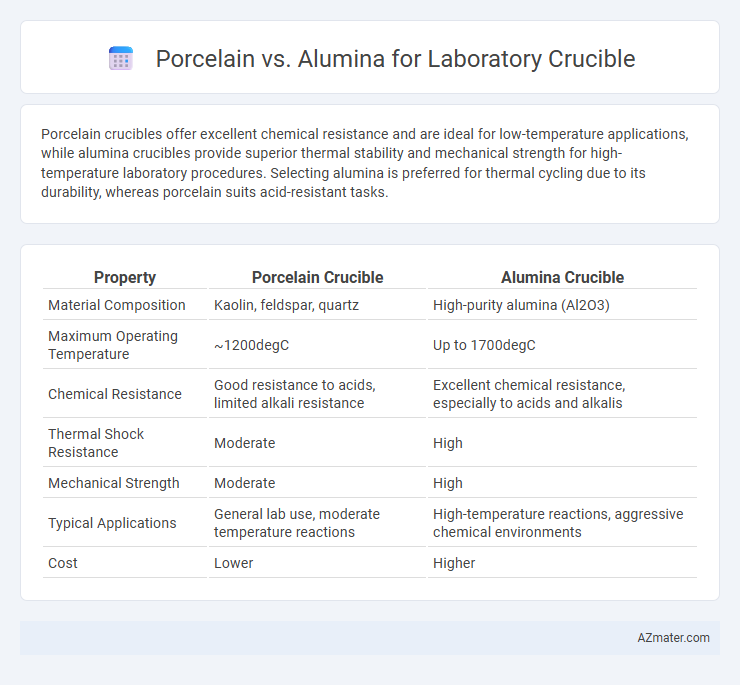
Porcelain crucibles offer excellent chemical resistance and are ideal for low-temperature applications, while alumina crucibles provide superior thermal stability and mechanical strength for high-temperature laboratory procedures. Selecting alumina is preferred for thermal cycling due to its durability, whereas porcelain suits acid-resistant tasks.
Table of Comparison
| Property | Porcelain Crucible | Alumina Crucible |
|---|---|---|
| Material Composition | Kaolin, feldspar, quartz | High-purity alumina (Al2O3) |
| Maximum Operating Temperature | ~1200degC | Up to 1700degC |
| Chemical Resistance | Good resistance to acids, limited alkali resistance | Excellent chemical resistance, especially to acids and alkalis |
| Thermal Shock Resistance | Moderate | High |
| Mechanical Strength | Moderate | High |
| Typical Applications | General lab use, moderate temperature reactions | High-temperature reactions, aggressive chemical environments |
| Cost | Lower | Higher |
Introduction to Laboratory Crucibles
Laboratory crucibles are essential tools used for high-temperature chemical analysis and material synthesis, designed to withstand extreme heat without deformation or contamination. Porcelain crucibles offer excellent resistance to thermal shock and chemical corrosion, making them suitable for general purpose applications involving moderate temperatures. Alumina crucibles, composed mainly of aluminum oxide, provide superior mechanical strength and higher temperature resistance, ideal for advanced analytical procedures requiring temperatures above 1600degC.
Overview of Porcelain Crucibles
Porcelain crucibles, made from refined kaolin clay, offer exceptional thermal stability and resistance to chemical corrosion, making them ideal for many laboratory applications involving high-temperature heating and strong acids. Their fine-grained structure provides durability and resistance to thermal shock, maintaining structural integrity during repeated heating cycles. Porcelain crucibles typically feature smooth surfaces that prevent contamination and facilitate easy cleaning, enhancing their suitability for precise analytical procedures.
Overview of Alumina Crucibles
Alumina crucibles, made from high-purity aluminum oxide, offer superior thermal stability and resistance to chemical corrosion compared to porcelain crucibles. These crucibles withstand temperatures exceeding 1700degC, making them ideal for high-temperature laboratory applications such as metal melting and powder analysis. Their non-porous, dense structure prevents contamination and ensures durability under aggressive chemical environments.
Chemical Resistance Comparison
Porcelain crucibles exhibit excellent chemical resistance to acids, alkalis, and salts, making them suitable for general lab applications involving mild to moderately aggressive chemicals. Alumina crucibles offer superior chemical resistance, particularly against strong acids, bases, and molten metals, due to their high purity and dense microstructure. This enhanced resistance makes alumina crucibles ideal for high-temperature processes and aggressive chemical environments where porcelain might degrade or react.
Thermal Stability and Maximum Temperature
Porcelain crucibles typically withstand maximum temperatures around 1,200degC, offering good thermal stability for many laboratory applications. Alumina crucibles provide superior thermal stability and can endure temperatures up to approximately 1,750degC, making them ideal for high-temperature processes. The higher melting point and thermal shock resistance of alumina make it preferable for applications requiring extreme heat and rapid temperature changes.
Mechanical Strength and Durability
Porcelain crucibles exhibit moderate mechanical strength, suitable for general laboratory use but prone to chipping under thermal shock. Alumina crucibles offer superior mechanical strength and enhanced resistance to wear, making them ideal for high-temperature applications requiring durability. Alumina's high fracture toughness ensures prolonged lifespan in rigorous processes compared to porcelain's more brittle nature.
Cost and Economic Considerations
Porcelain crucibles generally offer a lower upfront cost compared to alumina crucibles, making them a cost-effective choice for routine laboratory use with moderate temperature requirements. Alumina crucibles, though more expensive, provide superior thermal stability and chemical resistance, reducing replacement frequency and long-term operational expenses in high-temperature or corrosive environments. Economic considerations must balance initial investment against durability and application demands to optimize laboratory budget efficiency.
Typical Laboratory Applications
Porcelain crucibles are widely used for heating and melting chemical compounds due to their resistance to thermal shock and chemical corrosion, making them ideal for qualitative analysis and sample ashing. Alumina crucibles, offering higher mechanical strength and superior thermal stability at temperatures exceeding 1600degC, are preferred in high-temperature quantitative analysis and materials science experiments. Both materials serve critical roles in laboratory environments, with porcelain favored for routine analytical procedures and alumina chosen for demanding applications requiring enhanced durability and purity.
Maintenance and Care Requirements
Porcelain laboratory crucibles require careful handling to avoid chipping and must be cleaned with non-abrasive materials to maintain their smooth surface and thermal resistance. Alumina crucibles offer superior chemical inertness and thermal shock resistance, allowing for easier cleaning and less frequent replacement compared to porcelain. Both materials benefit from avoiding sudden temperature changes and thorough drying after washing to prolong their service life in laboratory applications.
Choosing the Right Crucible for Your Laboratory
Porcelain crucibles offer excellent resistance to thermal shock and chemical corrosion, making them ideal for general laboratory use involving moderately high temperatures and non-aggressive chemicals. Alumina crucibles provide superior mechanical strength and can withstand extremely high temperatures above 1700degC, suitable for applications requiring durability and resistance to chemical attack from strong acids and bases. Selecting the right crucible depends on the specific temperature range and chemical environment of your laboratory processes to ensure accuracy and longevity in your experiments.
Infographic: Porcelain vs Alumina for Laboratory Crucible
 azmater.com
azmater.com