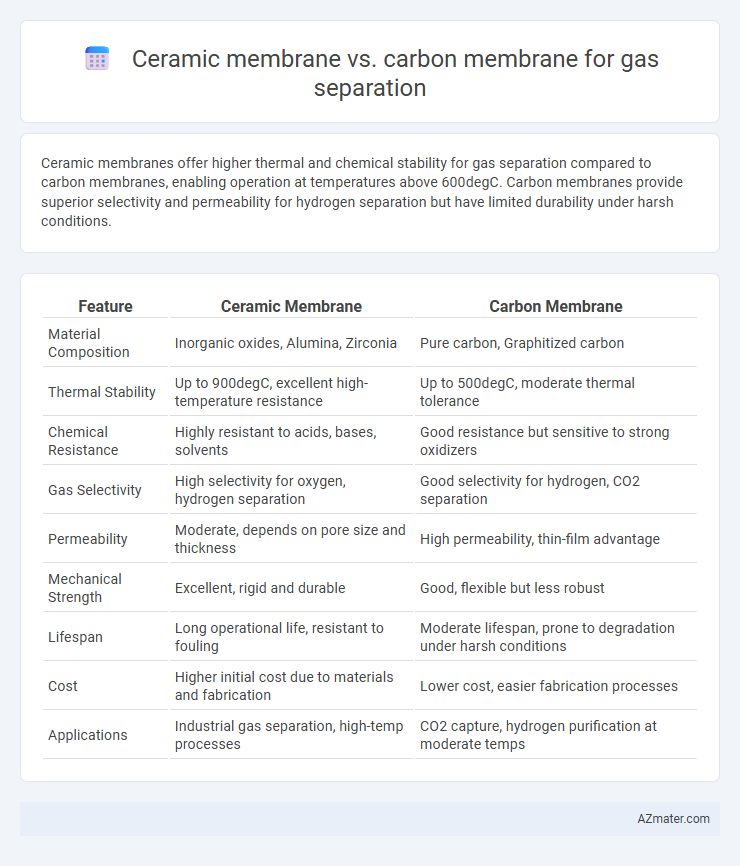
Ceramic membranes offer higher thermal and chemical stability for gas separation compared to carbon membranes, enabling operation at temperatures above 600degC. Carbon membranes provide superior selectivity and permeability for hydrogen separation but have limited durability under harsh conditions.
Table of Comparison
| Feature | Ceramic Membrane | Carbon Membrane |
|---|---|---|
| Material Composition | Inorganic oxides, Alumina, Zirconia | Pure carbon, Graphitized carbon |
| Thermal Stability | Up to 900degC, excellent high-temperature resistance | Up to 500degC, moderate thermal tolerance |
| Chemical Resistance | Highly resistant to acids, bases, solvents | Good resistance but sensitive to strong oxidizers |
| Gas Selectivity | High selectivity for oxygen, hydrogen separation | Good selectivity for hydrogen, CO2 separation |
| Permeability | Moderate, depends on pore size and thickness | High permeability, thin-film advantage |
| Mechanical Strength | Excellent, rigid and durable | Good, flexible but less robust |
| Lifespan | Long operational life, resistant to fouling | Moderate lifespan, prone to degradation under harsh conditions |
| Cost | Higher initial cost due to materials and fabrication | Lower cost, easier fabrication processes |
| Applications | Industrial gas separation, high-temp processes | CO2 capture, hydrogen purification at moderate temps |
Introduction to Gas Separation Technologies
Gas separation technologies utilize membranes made from diverse materials, with ceramic and carbon membranes being prominent options due to their distinct properties. Ceramic membranes offer high thermal and chemical stability, making them suitable for harsh gas separation environments, such as hydrogen recovery and carbon dioxide capture. Carbon membranes provide excellent permeability and selectivity for gases like oxygen and nitrogen, often favored in industrial applications requiring efficient air separation and gas purification.
Overview of Ceramic Membranes
Ceramic membranes for gas separation exhibit exceptional thermal and chemical stability, making them ideal for high-temperature and corrosive environments. These membranes utilize inorganic materials such as alumina, zirconia, and titania, offering superior durability compared to carbon membranes. Their rigid structure enables precise gas selectivity and permeance, crucial for industrial applications like hydrogen purification and flue gas treatment.
Overview of Carbon Membranes
Carbon membranes for gas separation offer high permeability and selectivity due to their unique microporous structure derived from the pyrolysis of polymer precursors. These membranes excel in separating gases like hydrogen, carbon dioxide, and hydrocarbons, benefiting from their thermal stability and chemical resistance. Compared to ceramic membranes, carbon membranes provide a cost-effective alternative with flexible fabrication options, although they often exhibit lower mechanical strength and long-term durability.
Key Mechanisms of Gas Separation
Ceramic membranes utilize molecular sieving and surface diffusion mechanisms, offering high thermal and chemical stability for selective gas separation, especially of hydrogen and oxygen. Carbon membranes rely on microporous structures enabling molecular sieving combined with solubility-diffusion, effectively separating gases like CO2 and N2. Ceramic membranes excel in harsh environments due to their robustness, while carbon membranes provide enhanced selectivity through tunable pore sizes and surface chemistry.
Performance Comparison: Permeability and Selectivity
Ceramic membranes exhibit higher thermal and chemical stability with superior selectivity for gas separation, especially in harsh environments, whereas carbon membranes generally offer higher permeability due to their porous structure. The selectivity of ceramic membranes is enhanced by their rigid, tunable pore sizes, making them suitable for separating gases like hydrogen and carbon dioxide with greater precision. Conversely, carbon membranes provide a balance between permeability and selectivity but may degrade faster under high temperatures or aggressive gas mixtures.
Thermal and Chemical Stability
Ceramic membranes exhibit superior thermal stability withstanding temperatures above 800degC, making them ideal for high-temperature gas separation applications. Their robust chemical resistance enables effective separation in harsh environments containing corrosive gases such as hydrogen sulfide or ammonia. Carbon membranes, while offering good selectivity, generally operate at lower temperatures and have limited resistance to oxidative degradation, restricting their use in extreme thermal or chemically aggressive conditions.
Fabrication and Cost Factors
Ceramic membranes for gas separation are fabricated using advanced techniques like sol-gel processing and phase inversion, resulting in high thermal and chemical stability but at a higher production cost due to expensive raw materials and energy-intensive processes. Carbon membranes are typically produced through pyrolysis of polymeric precursors, offering lower fabrication costs and good gas selectivity but limited thermal stability and shorter lifespan compared to ceramic membranes. Cost factors favor carbon membranes for low-budget or less demanding applications, while ceramic membranes justify their higher initial investment through durability and effective performance in harsh environments.
Common Applications in Industry
Ceramic membranes are widely used in high-temperature gas separation processes such as hydrogen purification and oxygen generation, owing to their thermal stability and chemical resistance. Carbon membranes excel in hydrocarbon separation and carbon dioxide capture due to their tunable pore structure and high permeability. Both membranes find critical applications in petrochemical refining, natural gas processing, and environmental control systems.
Environmental Impact and Sustainability
Ceramic membranes offer superior chemical stability and longer lifespan, reducing waste and environmental impact compared to carbon membranes, which are prone to fouling and shorter operational life. The inorganic composition of ceramic membranes enables higher-temperature operation and resistance to harsh gases, enhancing energy efficiency and sustainability in industrial gas separation processes. Carbon membranes, while cost-effective initially, present challenges in recycling and disposal, leading to higher ecological footprints relative to the more durable and recyclable ceramic alternatives.
Challenges and Future Perspectives
Ceramic membranes exhibit excellent thermal and chemical stability but face challenges related to high manufacturing costs and mechanical brittleness, limiting their large-scale application in gas separation. Carbon membranes offer high selectivity and permeability with better flexibility, yet they suffer from issues such as long-term stability and scalability in industrial environments. Future perspectives emphasize the development of hybrid membrane materials and advanced fabrication techniques to enhance durability, reduce costs, and improve performance for industrial gas separation processes.
Infographic: Ceramic membrane vs Carbon membrane for Gas separation
 azmater.com
azmater.com