Antimony exhibits high stability, dense atomic structure, and excellent semiconducting properties, making it ideal for electronic and material science research. Rubidium, a highly reactive alkali metal with low ionization energy, is primarily studied for its applications in quantum computing and atomic clock development.
Table of Comparison
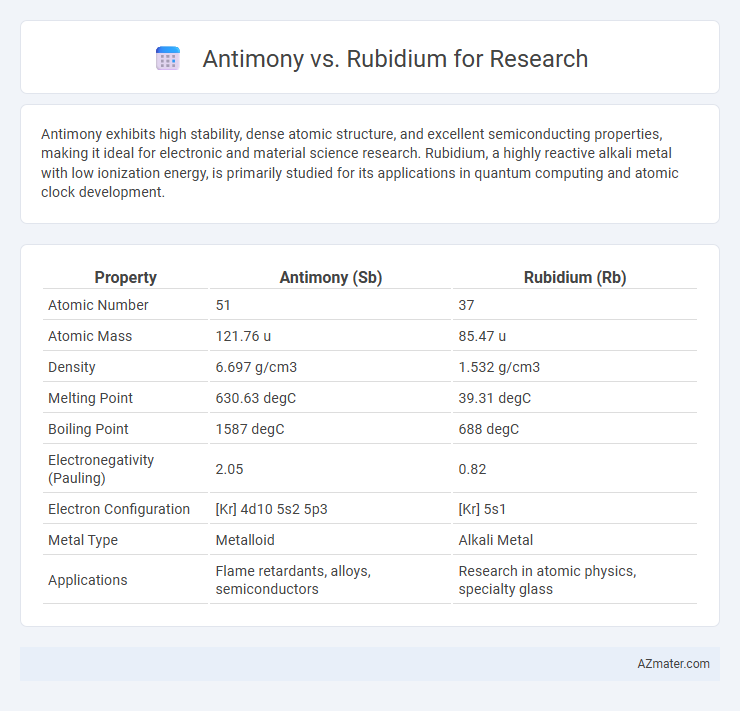
| Property | Antimony (Sb) | Rubidium (Rb) |
|---|---|---|
| Atomic Number | 51 | 37 |
| Atomic Mass | 121.76 u | 85.47 u |
| Density | 6.697 g/cm3 | 1.532 g/cm3 |
| Melting Point | 630.63 degC | 39.31 degC |
| Boiling Point | 1587 degC | 688 degC |
| Electronegativity (Pauling) | 2.05 | 0.82 |
| Electron Configuration | [Kr] 4d10 5s2 5p3 | [Kr] 5s1 |
| Metal Type | Metalloid | Alkali Metal |
| Applications | Flame retardants, alloys, semiconductors | Research in atomic physics, specialty glass |
Introduction to Antimony and Rubidium
Antimony, a metalloid with atomic number 51, is widely utilized in semiconductors, flame retardants, and alloys due to its unique electrical properties and chemical stability. Rubidium, an alkali metal with atomic number 37, is prized in research for its applications in atomic clocks, quantum mechanics, and spectroscopy because of its high reactivity and low ionization energy. Comparing these elements highlights Antimony's role in materials science and Rubidium's significance in precision measurement and quantum technology.
Elemental Properties Comparison
Antimony and rubidium differ significantly in elemental properties impacting research applications. Antimony (Sb), atomic number 51, is a metalloid known for its high density (6.697 g/cm3), semiconducting properties, and stability in compounds, making it valuable in electronics and flame retardants. Rubidium (Rb), atomic number 37, is an alkali metal with low density (1.532 g/cm3), high reactivity, and a low melting point (39.3degC), primarily used in atomic clocks and ion engines, highlighting contrasting physical and chemical behaviors essential for specialized scientific studies.
Abundance and Natural Occurrence
Antimony is moderately abundant in the Earth's crust, approximately 0.2 to 0.5 ppm, commonly found in sulfide minerals like stibnite, making it more accessible for research. Rubidium, although less abundant at about 90 ppm, occurs naturally in minerals such as lepidolite and pollucite, often in trace amounts within potassium minerals. The higher natural availability of antimony and its distinct mineral forms provide better prospects for extensive experimental studies compared to rubidium's scattered and lower concentration deposits.
Chemical Reactivity and Behavior
Antimony exhibits moderate chemical reactivity, primarily forming compounds such as antimonides and oxides, with a tendency to act as a metalloid in various reactions, displaying both metallic and non-metallic behavior. Rubidium, an alkali metal, is highly reactive, especially with water and halogens, forming rubidium hydroxide and rubidium halides rapidly, which is critical in studies involving strong reducing agents or ionic compounds. The stark contrast in their reactivity levels makes antimony suitable for research in semiconductor materials and alloy formation, while rubidium is extensively used in experiments requiring vigorous chemical interactions and ion-exchange processes.
Applications in Scientific Research
Antimony is widely used in semiconductor research and materials science due to its unique electrical properties and role as a dopant in the production of infrared detectors and thermoelectric devices. Rubidium finds significant applications in atomic physics and quantum research, especially in the development of atomic clocks, Bose-Einstein condensates, and precision spectroscopy. Both elements contribute to advanced experimental techniques but serve distinct purposes based on their chemical and physical characteristics in scientific investigations.
Handling and Safety Considerations
Antimony is a metalloid commonly used in semiconductor research, requiring careful handling due to its toxicity and potential for causing skin and respiratory irritation; lab personnel should use gloves and adequate ventilation to minimize exposure. Rubidium, an alkali metal, is highly reactive, especially with water, posing significant fire and explosion hazards; strict storage under inert atmospheres and the use of flame-resistant gloves and face shields are critical for safe manipulation. Both elements demand robust safety protocols, but rubidium's extreme reactivity necessitates more stringent precautions compared to antimony.
Analytical Techniques Involving Antimony and Rubidium
Antimony and rubidium serve distinct roles in analytical research, with antimony frequently analyzed through techniques such as atomic absorption spectroscopy (AAS), inductively coupled plasma mass spectrometry (ICP-MS), and X-ray fluorescence (XRF) due to its toxicological and industrial relevance. Rubidium, on the other hand, is commonly measured using mass spectrometry and flame emission spectroscopy to study its geochemical and biological functions. The choice of analytical method depends on factors like sensitivity requirements, sample matrix, and concentration levels specific to antimony and rubidium.
Environmental Impact Assessment
Antimony exhibits moderate environmental toxicity due to its persistence and bioaccumulation potential, necessitating careful monitoring in research involving soil and water contamination assessments. Rubidium, with relatively low natural abundance and minimal toxicity, poses less environmental risk but requires analysis of its geochemical behavior to understand long-term ecological impacts. Comprehensive environmental impact assessments must consider antimony's heavy metal characteristics and rubidium's alkali metal properties to effectively evaluate their ecological risks in research settings.
Cost and Accessibility for Laboratories
Antimony offers a cost-effective option for laboratories due to its abundant availability and lower market price compared to rubidium, which is relatively rare and expensive. Rubidium's higher cost and limited accessibility can restrict its use primarily to specialized research requiring its unique alkali metal properties. Laboratories prioritizing budget and ease of procurement often opt for antimony, while those focusing on advanced applications involving rubidium's distinct chemical characteristics might justify the higher expense.
Future Prospects in Material Science Research
Antimony exhibits promising potential in material science research due to its applications in semiconductors, thermoelectric devices, and advanced alloys, driven by its unique electronic properties and abundance. Rubidium, with its exceptional alkali metal characteristics and significance in quantum computing and atomic clocks, opens future prospects for developing innovative materials and ultra-sensitive sensors. Both elements are critical to advancing next-generation technologies, with antimony focusing on energy-efficient materials and rubidium on quantum-based applications.
Infographic: Antimony vs Rubidium for Research
 azmater.com
azmater.com